How Poor Sleep Accelerates Brain Aging: The Science Behind Sleep Deprivation's Impact on Your Brain [2025]
You know that feeling after a bad night of sleep—when your brain feels foggy and your body feels heavy? That's not just a temporary inconvenience. That's your brain literally aging faster.
Here's what recent neuroscience research is revealing: people who sleep poorly don't just feel older. Their brains actually are older. We're talking about measurable differences in brain structure and function that stack up over time, turning a few rough nights into what amounts to years of accelerated cognitive decline.
The research is striking. Scientists found that for every point decrease in sleep quality on their measurement scale, people's brains aged about six months faster. For those with the poorest sleep, their brains were roughly one year older than their actual chronological age. That's not a rounding error. That's a significant, quantifiable gap that compounds year after year.
What's particularly revealing is that researchers have figured out the mechanisms driving this process. It's not just one thing. It's a cascade of biological events triggered by sleep deprivation: chronic inflammation spreading through your body, toxic waste accumulating in your brain because the cleanup crew isn't running the night shift, and cardiovascular damage that starves your brain of oxygen-rich blood.
The question isn't whether poor sleep matters anymore. The science is settled on that front. The real question now is: what exactly is happening in your brain when you sleep poorly, and what can you actually do about it?
Let's walk through the research, break down the mechanisms, and figure out what this means for your sleep tonight and your brain health over the next decade.
TL; DR
- Brain Aging: Poor sleep quality makes your brain biologically 1 year older than your chronological age, with 6 months of aging per quality-point decrease
- Inflammation Connection: Low-grade chronic inflammation explains 7-10% of the association between poor sleep and accelerated brain aging
- Lifestyle Factors: Night-owl chronotype, unhealthy sleep duration (beyond 7-8 hours), and snoring are the strongest predictors of brain aging
- Prevention Strategy: Maintaining consistent sleep schedules, targeting 7-8 hours nightly, and addressing sleep disorders can slow brain aging significantly
- Bottom Line: Sleep quality is one of the few modifiable factors that directly impacts how fast your brain ages

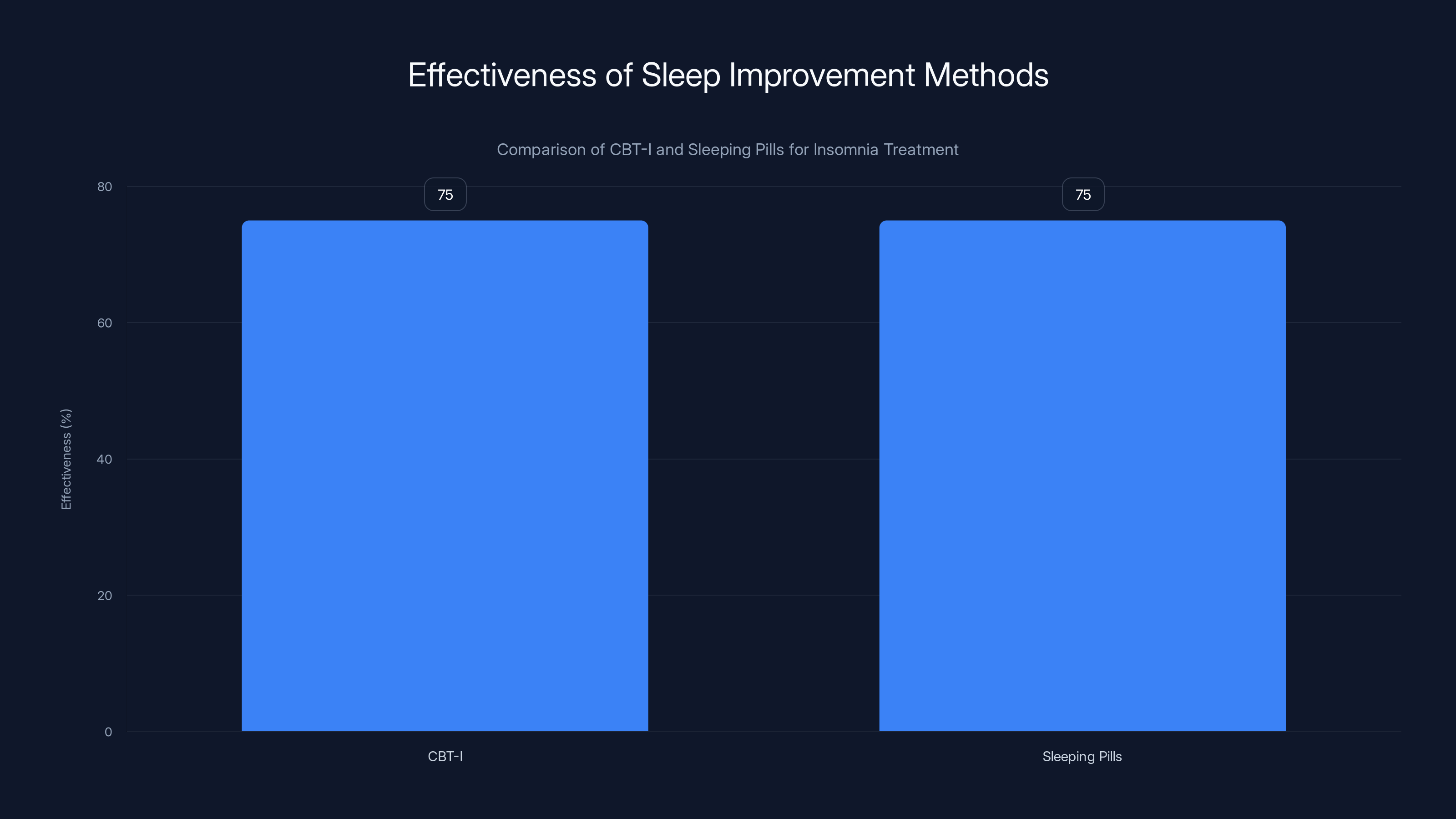
CBT-I and sleeping pills both have an effectiveness rate of approximately 75% for treating insomnia, but CBT-I offers better long-term outcomes without side effects. Estimated data based on typical effectiveness rates.
The Research That Changed Everything: Understanding Brain Age vs. Chronological Age
For years, scientists knew that poor sleep and dementia were connected. But they couldn't untangle cause from effect. Did poor sleep cause dementia, or was poor sleep an early symptom of dementia already developing? It's a crucial distinction that completely changes how we think about prevention.
New research from the Karolinska Institute in Sweden provided the breakthrough. Researchers studied over 27,500 middle-aged and elderly people from the UK Biobank, a massive research database tracking genetic predisposition and lifestyle factors across hundreds of thousands of people. The average age of participants was 54.7 years.
Here's what made this study different. Instead of just asking people whether they slept well, researchers developed a multi-dimensional assessment of sleep quality. They looked at five separate factors: your chronotype (whether you're a morning person or night owl), how long you actually sleep, whether you have insomnia, whether you snore, and how sleepy you feel during the day. This gave them a comprehensive picture of sleep patterns, not just a yes-or-no assessment.
About nine years after the initial sleep assessment, researchers scanned all these participants' brains with MRI. But here's where it gets sophisticated. They didn't just look at brain scans visually. They used machine learning models—AI trained on thousands of brain scans—to estimate each person's biological brain age. Think of it like a model that's learned the patterns of how brains actually age, then applies that knowledge to estimate where any individual brain falls on that aging timeline.
Then came the key finding: the researchers compared each person's biological brain age (what the AI calculated) to their actual chronological age. The gap between these numbers revealed something profound.
For every single point decrease on their sleep quality scale, the difference between brain age and chronological age increased by approximately six months. In practical terms, that means one shift in your sleep pattern measurements equals half a year of accelerated brain aging. The people with the worst sleep? Their brains were approximately one year older than they actually were.
Abigail Dove, the neuroepidemiologist leading the research, explained it plainly: "Our findings provide evidence that poor sleep may contribute to accelerated brain aging, and point to inflammation as one of the underlying mechanisms."
This is crucial because it moves sleep from the "nice to have" category into the "actually protects your brain structure" category.
Sleep Quality Dimensions: What Actually Gets Measured
When researchers talk about "sleep quality," they're not referring to something vague. They're measuring five specific, observable factors that together paint a complete picture of sleep patterns.
Understanding these five dimensions matters because they interact with each other. Improving one can help improve another. And some are more strongly linked to brain aging than others.
Chronotype: Morning Person vs. Night Owl
Your chronotype is determined largely by genetics and your circadian rhythm—the internal clock that makes you naturally alert at certain times and sleepy at others. Some people are naturally wired to wake at 5 AM feeling refreshed. Others don't hit their stride until 10 PM.
Here's where it gets problematic: modern society runs on a morning-person schedule. Schools start early. Offices open at 9 AM. If you're a natural night owl trying to wake up at 6 AM, you're fighting your biology every single day.
The research found that people with a "night owl" chronotype showed significantly more brain aging. This isn't because being a night owl is inherently bad. It's because night owls forced into a morning schedule experience chronic circadian misalignment. Their body is telling them to sleep at 2 AM, but they need to be awake at 6 AM for work.
This chronic conflict between your internal clock and external demands creates stress on your system. Your cortisol levels stay elevated. Your sleep, even when it finally happens, is shallower and more disrupted. The cumulative effect: accelerated brain aging.
Interestingly, if a night owl can work a schedule that aligns with their natural rhythm, they can avoid this penalty. The problem isn't the chronotype itself. It's the mismatch.
Sleep Duration: The Goldilocks Zone
Everyone knows that sleeping too little is bad. Seven or eight hours per night is the standard recommendation. But the research revealed something surprising: sleeping significantly more than 8-9 hours per night is also associated with brain aging.
This creates a specific optimal range. The research identified 7-8 hours as ideal. Less than that, and you're not giving your brain enough time for essential maintenance. More than that consistently, and it suggests underlying sleep problems—like sleep apnea causing fragmented sleep, or depression causing oversleeping.
Think about what happens when someone consistently sleeps 10-11 hours per night. That's usually not because they love sleep. It's often because their sleep quality is so poor that they need more quantity to feel even slightly rested. Or it indicates a health problem that itself accelerates aging.
The key insight: it's not about maximizing sleep. It's about finding your individual sweet spot, typically in that 7-8 hour range.
Insomnia: The Cascade of Sleep Disruption
Insomnia isn't just about not being able to fall asleep. The research measured several aspects: difficulty falling asleep, staying asleep, early morning awakening, and non-restorative sleep (waking up feeling unrefreshed despite "sleeping").
What's particularly interesting is how insomnia cascades into other sleep problems. Someone with insomnia lies awake at night, becomes anxious about sleep itself, and then experiences daytime sleepiness because they're so sleep-deprived. That daytime sleepiness might then make it harder to stay awake when they need to be alert, causing them to nap in the afternoon, which then makes falling asleep at night even harder.
The brain aging signal is strongest here because insomnia creates persistent sleep fragmentation. Your brain isn't getting the consolidated, deep sleep it needs for essential maintenance processes.
Snoring: A Sign of Deeper Sleep Disruption
Snoring gets a lot of jokes. It's treated as an inconvenience for sleep partners. But from a neuroscience perspective, snoring is a red flag.
Snoring often indicates that breathing is being partially obstructed during sleep. Your airway isn't completely blocked (that would be full sleep apnea, which is even worse), but it's narrowed. Your body keeps partially waking to gasp for air. You might not consciously remember these awakenings—there can be dozens or hundreds per night—but your brain does.
This partial obstruction means your blood oxygen saturation dips repeatedly through the night. Your brain isn't getting the oxygen it needs. Over time, this chronic oxygen deprivation damages brain tissue.
People who snore showed some of the strongest associations with brain aging in the research, which suggests that many snorers actually have undiagnosed sleep apnea.
Daytime Sleepiness: The Exhaustion Signal
Daytime sleepiness indicates that whatever sleep you're getting isn't restorative. You might technically be "asleep" for eight hours, but if you wake up exhausted and can barely stay awake at your desk by 2 PM, something's wrong.
This symptom often indicates fragmented sleep or insufficient deep sleep. Your brain didn't get the recovery it needed during the night.

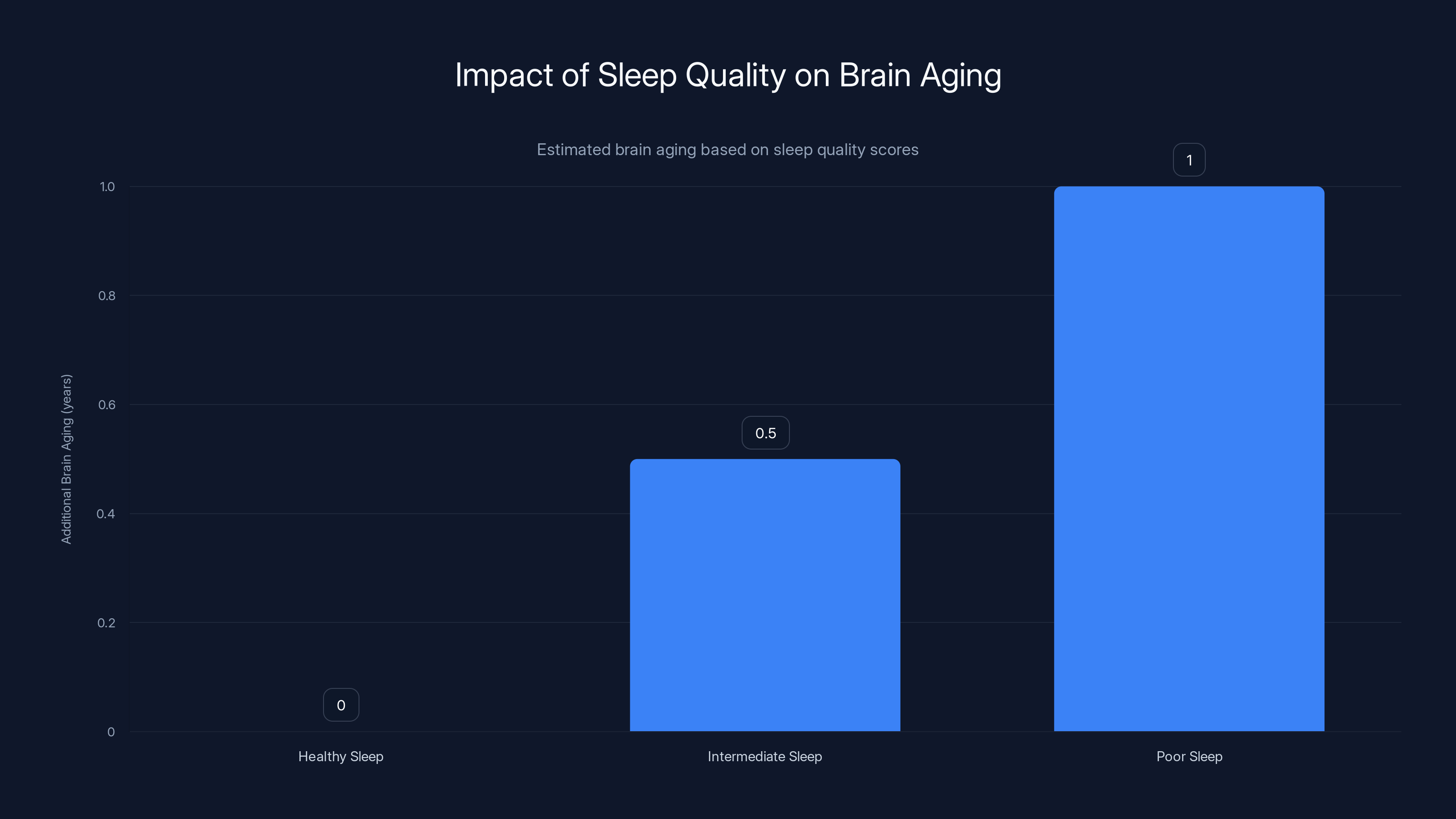
Poor sleep quality significantly accelerates brain aging, with the poorest sleepers experiencing an additional year of brain aging compared to their chronological age. Estimated data based on research findings.
The Brain Aging Connection: How the Math Works
Let's make the research concrete. Researchers created a "sleep quality score" based on these five dimensions. Lower scores meant worse sleep. Then they looked at what happened to biological brain age as this score declined.
The relationship was linear and dose-dependent. Here's the math:
For every 1-point decrease in sleep quality score = approximately 6 months of additional brain aging
If sleep quality drops by 2 points, that's a year of accelerated aging. By 3 points, eighteen months. This relationship held up across the entire population they studied.
But the really striking finding was the extremes. Researchers categorized sleep quality into three groups:
- 41.2% had healthy sleep (high quality across all five dimensions)
- 55.6% fell into an intermediate group (problems in some areas, okay in others)
- 3.3% had clearly poor sleep (problems across multiple dimensions)
That smallest group—people with poor sleep across the board—showed approximately one year of brain aging beyond their chronological age. A 60-year-old in this group had the brain of a 61-year-old. A 50-year-old had the brain of a 51-year-old.
Over a lifetime, this compounds. A person who maintains poor sleep from age 40 to 70 could end up with a brain that's biologically equivalent to someone in their 80s.
The Inflammation Hypothesis: Understanding the Mechanism
Knowing that poor sleep accelerates brain aging was important. But the real breakthrough was understanding why. Researchers hypothesized that chronic inflammation was the link.
Inflammation is your body's immune response to threats. Acute inflammation—your ankle swelling after a sprain—is protective and temporary. Chronic low-grade inflammation is different. It's persistent, systemic, and damaging. It's your immune system in a constant state of low-level activation, treating your own tissues as problems to solve.
Poor sleep creates conditions for chronic inflammation. When you don't sleep enough or sleep poorly, your immune system becomes dysregulated. Inflammatory markers in your blood increase. Your body starts showing signs of being in a state of immune activation.
To measure this, researchers analyzed multiple inflammatory biomarkers:
- C-reactive protein (CRP): Produced by the liver in response to inflammation. Elevated CRP is linked to numerous diseases
- White blood cell and platelet counts: Changes in these indicate immune activation
- Granulocyte-to-lymphocyte ratio: A specific immune marker that shifts when the immune system is actively responding
The results confirmed the hypothesis: higher inflammation levels correlated with increased brain age.
But here's where mediation analysis became crucial. Mediation analysis is a statistical technique that asks: "How much of the effect of A on C is explained by B?" In this case: "How much of poor sleep's effect on brain aging is explained by inflammation?"
The findings:
- For intermediate sleep quality: Inflammation explained approximately 7% of the association with brain aging
- For poor sleep quality: Inflammation explained more than 10% of the association with brain aging
This means inflammation is one important mechanism, but it's not the whole story. About 90% of the brain aging effect from poor sleep works through other pathways.

The Glymphatic System: Your Brain's Nighttime Cleanup Crew
While inflammation explains part of the story, researchers identified another crucial mechanism: the glymphatic system.
Your brain produces toxic waste products constantly. Proteins misfold. Cellular debris accumulates. Metabolic byproducts build up. During waking hours, your brain is too busy processing information to clean house. But during sleep, something remarkable happens.
Glial cells—support cells in your brain that aren't neurons—actively pump cerebrospinal fluid through brain tissue. This fluid washes through your brain like a dishwasher, flushing out waste products. This system is called the "glymphatic system" because it works similarly to the lymphatic system in your body, but for the brain (hence "glial" + "lymphatic").
One of the primary waste products the glymphatic system removes is beta-amyloid. This protein tends to misfold and accumulate in Alzheimer's disease. Poor sleep means your glymphatic system isn't running efficiently. Beta-amyloid and other toxins build up.
Over time, accumulated toxic proteins damage neurons. They interfere with communication between brain cells. They trigger inflammatory cascades. The result: cognitive decline.
This mechanism is particularly important because it explains why sleep deprivation is so specifically damaging to brain health. It's not just about feeling tired. Your brain quite literally accumulates poison when you don't sleep.
MRI studies have actually visualized this happening. When people sleep, their brain cells shrink by about 60%, making space for cerebrospinal fluid to flow through and clean. When they don't sleep, this clearance doesn't happen.
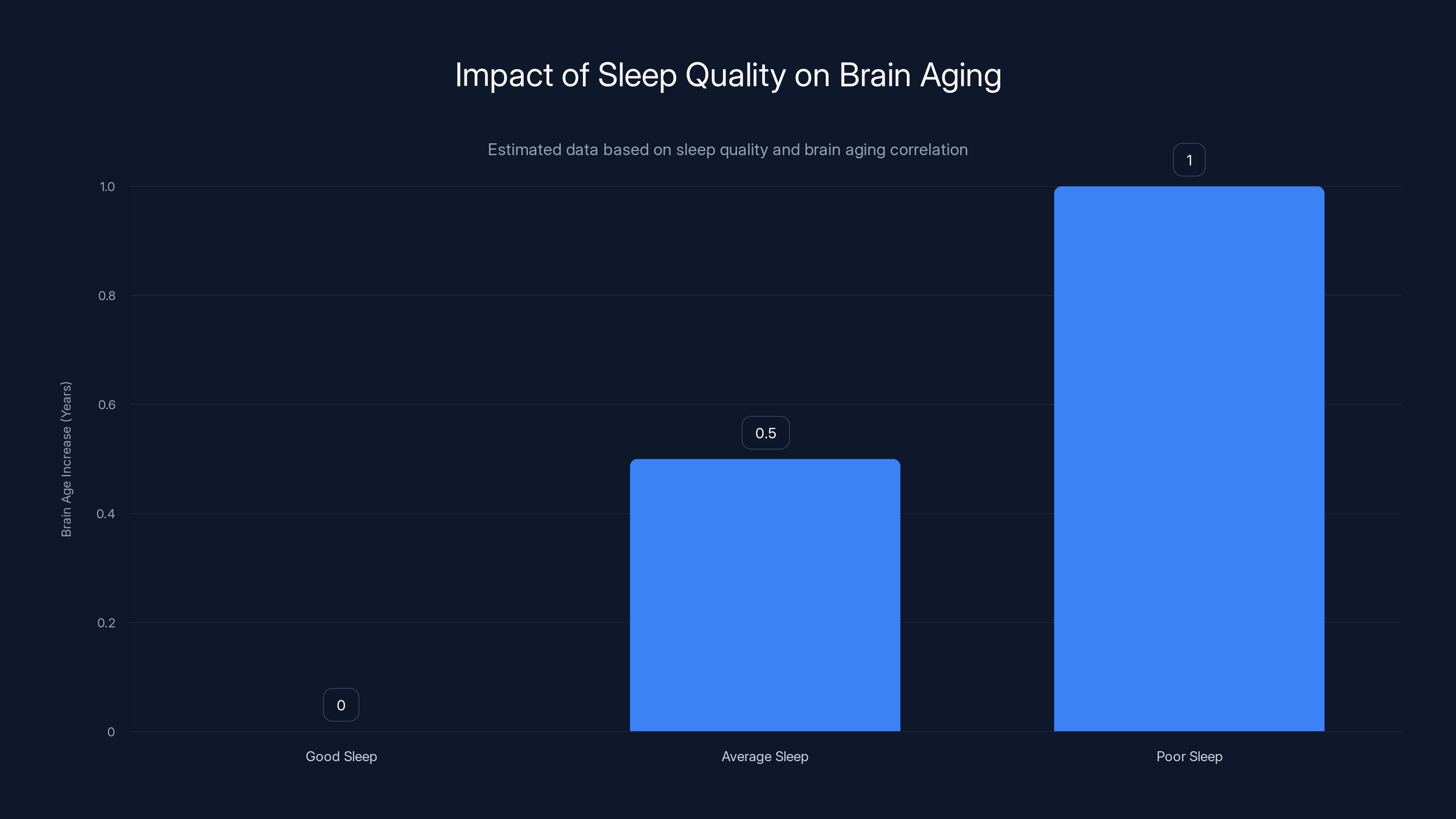
Poor sleep quality can make your brain biologically older by up to 1 year compared to your chronological age. Estimated data based on the correlation between sleep quality and brain aging.
Cardiovascular Complications: How Poor Sleep Damages Blood Flow to Your Brain
The brain represents only about 2% of your body weight but demands about 15-20% of your blood flow. It's extraordinarily vascular and oxygen-demanding.
Poor sleep damages cardiovascular health in multiple ways:
Blood Pressure Dysregulation: During sleep, your blood pressure normally drops by 10-20%. This dipping is protective—it gives your cardiovascular system a rest. Poor sleep flattens this dipping pattern. Your blood pressure stays elevated even at night. Over time, chronically elevated blood pressure damages the endothelium—the inner lining of blood vessels.
Arterial Stiffness: Chronic poor sleep accelerates the development of arterial stiffness. Your blood vessels become less flexible and more prone to plaques and blockages. Reduced blood flow to the brain means less oxygen reaching brain tissue.
Atherosclerosis Progression: Poor sleep is associated with accelerated development of atherosclerosis—plaque buildup in arteries. In the brain, this reduces blood flow to specific regions.
Increased Clotting Risk: Sleep deprivation increases platelet activation and makes blood more prone to clotting. A clot could block blood flow to brain regions, causing a stroke.
The net result: your brain is getting less oxygen and fewer nutrients than it needs. Brain cells are forced to function in a state of mild hypoxia (oxygen deprivation). Over time, this damages cells and accelerates aging.
MRI studies show that people with poor sleep have increased white matter hyperintensities—signs of damage to the brain's white matter tracts that carry signals between regions. These hyperintensities are associated with cognitive decline and dementia risk.
Night Owls vs. Morning People: Chronotype Mismatch and Brain Aging
The research highlighted that chronotype—whether you're naturally a morning person or night owl—significantly predicts brain aging. But the important caveat is "mismatch."
Your chronotype is largely determined by genetics. Studies show that about 50% of the variation in chronotype between people is heritable. There's a clock gene in your cells (PER1, PER2, PER3 genes) that influences your natural sleep-wake cycle.
But modern society runs on a "tyranny of the morning." Schools start early. Work typically starts at 9 AM. Evening social obligations push sleep even later. For about 40% of the population with a natural evening preference, this creates chronic conflict.
A night owl naturally gets alert around 10-11 PM and would sleep 1-2 AM to 9-10 AM. But work demands they be alert at 9 AM. So they set an alarm for 6 AM and sleep 1-2 AM to 6 AM. That's 4-5 hours of fragmented sleep when their body temperature is supposed to be dropping and they should be entering deep sleep.
The research found that night owls showed greater brain aging. But here's the key: this wasn't inevitable. Night owls who could structure their schedule around their natural rhythm didn't show this acceleration. The problem was the mismatch.
This has important implications. Some people benefit more from sleep optimization strategies than others. A natural early riser might sleep 7-8 hours with relative ease. A night owl with the same 7-8 hour opportunity might get much lower quality sleep because it's happening during their biological "wake time."
The implication: matching your sleep schedule to your natural chronotype, when possible, is a powerful way to improve sleep quality and potentially slow brain aging.
Snoring and Sleep Apnea: When Breathing Becomes the Problem
Snoring is often dismissed as a minor inconvenience. Joke about it, nudge your partner, move on. But from a neurological perspective, snoring is a serious warning sign.
Snoring occurs when tissues in your airway vibrate as air passes through. The tighter the airway, the louder the snoring. But importantly, snoring indicates airway resistance and partial obstruction.
In some cases, the obstruction becomes complete. Your airway fully closes, breathing stops for 10-120 seconds, your oxygen saturation drops, and then your brain jolts you slightly awake to start breathing again. This is sleep apnea.
Even without full apnea, chronic snoring means repeated partial obstruction. Your brain experiences dozens or hundreds of micro-arousals—brief awakenings—per night. You might not consciously remember them, but they fragment your sleep.
The oxygen deprivation is particularly damaging to the brain. Neurons are extraordinarily oxygen-dependent. The hippocampus—crucial for memory formation—is especially vulnerable to hypoxic damage.
People with sleep apnea have dramatically increased dementia risk. They have more brain atrophy (brain tissue loss). They accumulate beta-amyloid faster. MRI studies show white matter damage comparable to people 10-15 years older.
In the research, snoring was one of the strongest predictors of brain aging. And it's one of the most actionable items. Sleep apnea is treatable. CPAP machines deliver continuous positive airway pressure, keeping the airway open. With treatment, much of the brain damage risk is reversible.
If you snore, getting a sleep study is worth prioritizing. The potential benefit to your brain health is substantial.

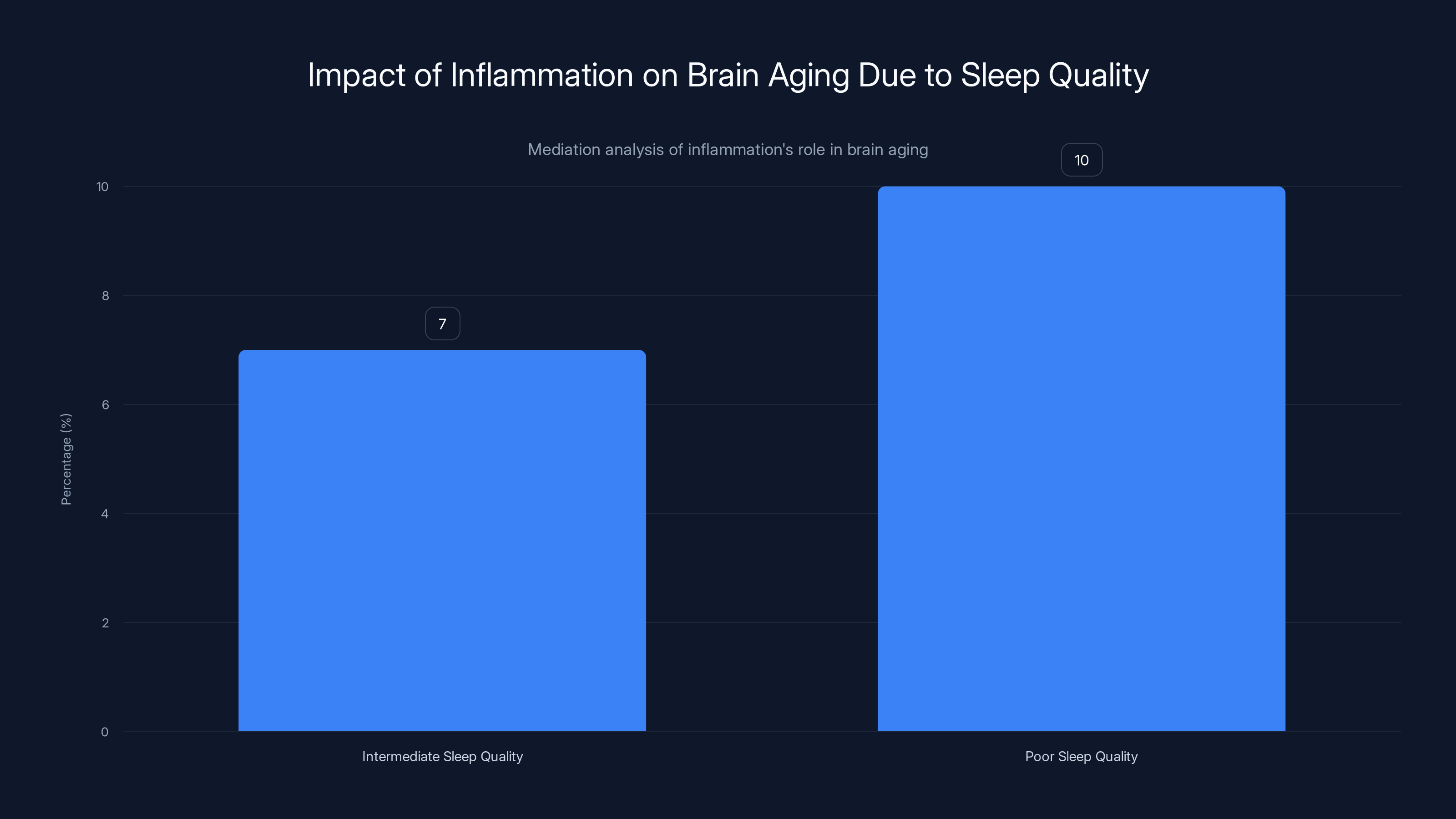
Inflammation explains 7% of brain aging in intermediate sleep quality and over 10% in poor sleep quality, highlighting its significant role.
Insomnia: When Your Brain Won't Turn Off
Insomnia affects about 30-35% of the population, and it's strongly associated with brain aging in the research.
Insomnia isn't simple. It comes in different forms:
Sleep Onset Insomnia: You can't fall asleep. You lie in bed for 30-60+ minutes before sleep comes. Often driven by racing thoughts, anxiety, or hyperarousal.
Sleep Maintenance Insomnia: You fall asleep fine, but wake repeatedly during the night. You might wake to use the bathroom, or just wake for no clear reason and can't fall back asleep.
Early Morning Awakening: You wake at 3-4 AM and can't get back to sleep. Often associated with depression and aging.
Non-Restorative Sleep: You sleep seven or eight hours, but wake up feeling unrefreshed, like you didn't actually sleep. The sleep quality is so poor that quantity doesn't help.
These different forms sometimes have different causes:
- Sleep onset insomnia is often driven by anxiety and hyperarousal (your nervous system is too activated)
- Sleep maintenance insomnia can be caused by sleep apnea, restless leg syndrome, or frequent bathroom trips
- Early morning awakening is often linked to depression or age-related circadian changes
- Non-restorative sleep might indicate poor sleep architecture—not enough deep sleep or REM sleep
What they all have in common: fragmented, light, insufficient sleep. Your brain doesn't get enough consolidated time in deep sleep stages where memory consolidation and toxin clearance happen.
The brain aging signal is strong because insomnia creates chronic sleep fragmentation. Unlike sleep apnea where the problem is breathing, insomnia is a problem with sleep itself. And unlike lifestyle issues where the solution is behavior change, insomnia often requires treatment—therapy, medication, or both.
Daytime Sleepiness: When Sleep Isn't Working
Daytime sleepiness is different from normal tiredness. It's an inability to maintain wakefulness during the day, even when you're doing something that should be engaging.
Someone with daytime sleepiness might fall asleep during meetings, while watching TV, or even while driving. This isn't laziness. It indicates insufficient or poor-quality sleep at night.
Daytime sleepiness can stem from:
- Insufficient sleep duration: Simply not getting enough hours
- Fragmented sleep: Sleep apnea, periodic leg movements, or frequent awakenings
- Circadian misalignment: Your sleep is happening at the wrong time relative to your internal clock
- Poor sleep quality: Not spending enough time in deep sleep stages
- Underlying conditions: Narcolepsy, hypersomnia, depression, or thyroid problems
When daytime sleepiness appears alongside other sleep problems, it signals that your brain isn't getting sufficient restorative sleep. Your neurons aren't consolidating memories properly. Your glymphatic system isn't clearing toxins effectively.
The research found that daytime sleepiness was one of the five strong predictors of brain aging. It's often a sign that one or more of the other sleep problems is present.

The Compounding Effect: How Sleep Problems Interact
One of the most important findings from the research was that these five sleep dimensions don't operate independently. They interact and compound.
For example, someone with a night-owl chronotype who's forced into a morning schedule might develop insomnia (can't fall asleep at 10 PM when work requires a 6 AM wake-up). The insomnia leads to sleep deprivation and fragmented sleep. Fragmented sleep leads to daytime sleepiness. The daytime sleepiness might lead to napping in the afternoon, which then makes falling asleep at night even harder.
Or someone with sleep apnea snores and experiences repeated breathing interruptions. This fragments their sleep, so even though they're "asleep" 8 hours, the quality is terrible. They wake unrefreshed, feel exhausted during the day, and their sleep duration might be reduced because the poor quality makes them wake earlier.
Each problem amplifies the others. Someone with poor sleep typically isn't just dealing with one issue. They're dealing with a constellation of problems that feed into each other.
This has important implications for treatment. Addressing one issue might help several others. Someone with sleep apnea who gets treated with CPAP might find their insomnia improves. Someone who shifts their work schedule to match their chronotype might find sleep apnea symptoms diminish (chronotype misalignment can worsen sleep apnea).
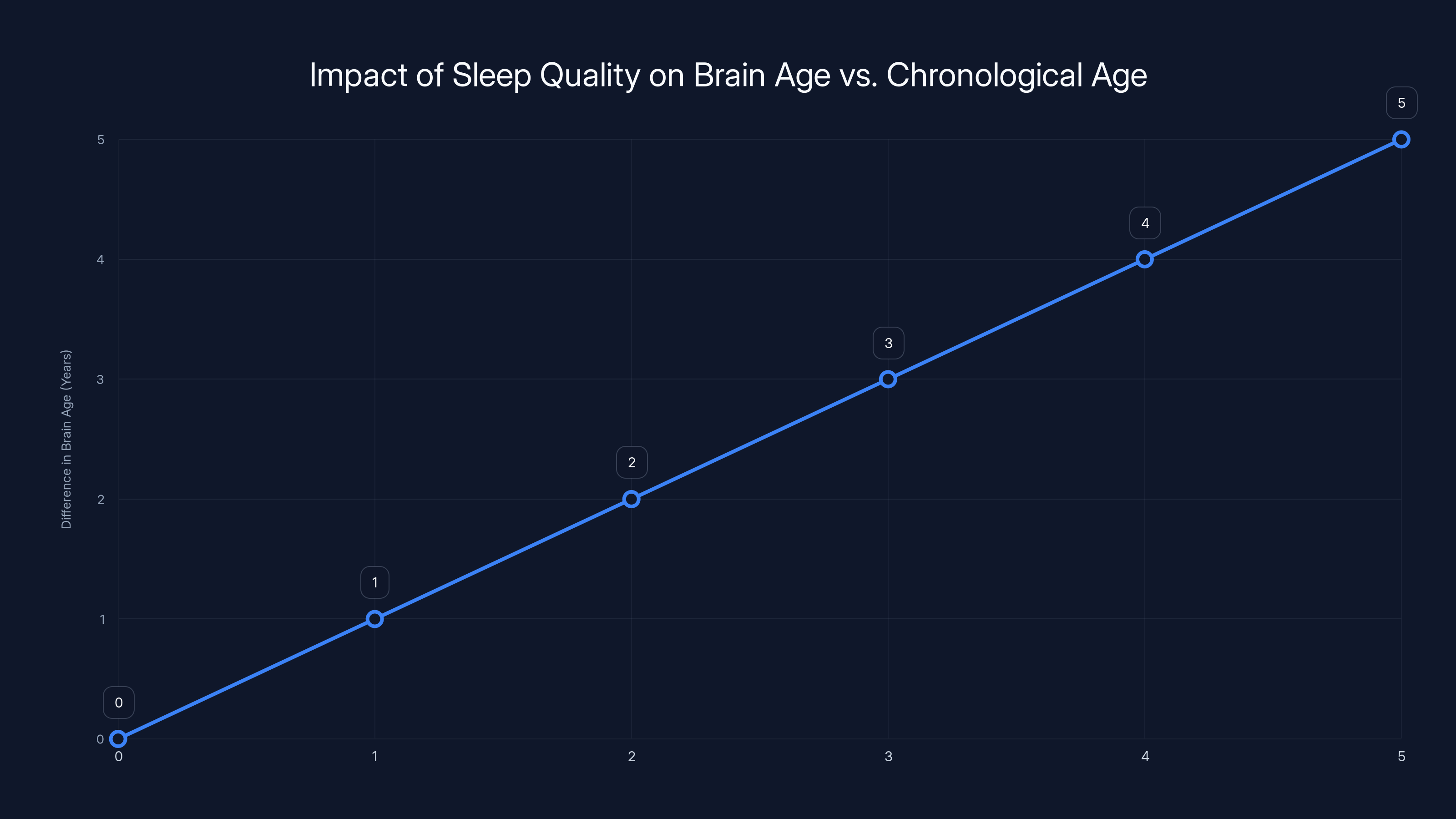
Estimated data suggests that for each point decrease in sleep quality, the difference between brain age and chronological age increases by approximately one year, highlighting the significant impact of sleep on brain aging.
Measurable Brain Changes: What the MRI Shows
The brain aging findings from MRI aren't just statistical artifacts. Researchers can actually see physical differences in brains of people with poor sleep versus those with good sleep.
People with poor sleep show several structural differences:
Reduced Gray Matter Volume: Particularly in the hippocampus (crucial for memory), prefrontal cortex (decision-making and impulse control), and anterior cingulate cortex (emotion regulation). These regions are smaller, with fewer neurons.
White Matter Hyperintensities: Bright spots visible on MRI indicating damage to white matter—the brain's communication cables. These are associated with cognitive decline and dementia risk.
Reduced Cortical Thickness: The gray matter layer at the brain's surface is thinner, suggesting neuronal loss.
Impaired Connectivity: Brain regions that normally communicate well show reduced functional connectivity. It's like the brain's internal network is degrading.
Increased Ventricular Size: The fluid-filled chambers in the brain's center expand, a sign of brain tissue loss.
These aren't subtle changes. They're measurable, consistent, and associated with cognitive problems in real life. People showing these changes perform worse on memory tests, processing speed tests, and executive function tests.
The encouraging part: these changes appear to be at least partially reversible. When people improve their sleep, brain changes begin to normalize. This suggests that sleep deprivation causes damage, but not necessarily permanent, irreversible damage—if addressed.
Age-Related Acceleration: Why This Matters More as You Get Older
While the brain aging effect of poor sleep is present across the age range studied (the cohort averaged 54.7 years), the impact becomes more significant as people age.
This is because aging itself accelerates brain aging. After about age 60, the brain naturally begins to shrink and cognitive function naturally begins to decline. For people in their 60s, 70s, or 80s, adding accelerated brain aging from poor sleep on top of age-related decline creates compounding damage.
A healthy 70-year-old sleeps well and might have a brain age of 71-72. A 70-year-old with poor sleep might have a brain age of 73-75. That 3-5 year gap might be the difference between maintaining independence and developing cognitive decline.
This is also why improving sleep becomes increasingly important as you age. In your 20s or 30s, poor sleep damages your brain, but you have the capacity to repair most of that damage. In your 70s, your brain's repair capacity is diminished. The damage from poor sleep is harder to reverse and more consequential.
The research suggests that protecting sleep quality becomes increasingly critical as a preventative health measure as you age.
Prevention: Optimizing Sleep for Brain Health
The research establishes that poor sleep accelerates brain aging. The logical next question: what actually works to improve sleep?
Behavioral Sleep Medicine
Cognitive Behavioral Therapy for Insomnia (CBT-I) is the gold standard, evidence-based treatment for insomnia. It works by addressing the thoughts and behaviors that maintain insomnia.
CBT-I typically includes:
Sleep Restriction: Limiting the time you spend in bed to only the time you actually sleep. If you're in bed 9 hours but sleeping 6, you restrict your time in bed to 6-6.5 hours initially. This consolidates sleep and reduces the wakeful time spent in bed worrying about not sleeping.
Stimulus Control: Using your bed only for sleep (and sex). Not working, eating, or watching TV in bed. This re-establishes the brain's association between bed and sleep.
Cognitive Restructuring: Identifying thoughts that fuel insomnia ("I'll never fall asleep," "I need 8 hours or I'll be exhausted") and examining evidence for and against these thoughts.
Sleep Hygiene: The basics—cool, dark, quiet bedroom; consistent sleep-wake times; limiting caffeine and alcohol; avoiding screens before bed.
Worry Time: Scheduling a specific time during the day to worry about problems, so you're not ruminating at 2 AM.
Research shows CBT-I has about 70-80% effectiveness for insomnia, comparable to sleeping pills but with better long-term outcomes and no side effects.
Sleep Apnea Treatment
For sleep apnea, the first-line treatment is CPAP—a small mask that delivers positive airway pressure, keeping your airway open during sleep. It sounds uncomfortable but most people adapt within a couple of weeks.
The benefit to brain health is substantial. Within weeks of starting CPAP, people often report improved alertness and cognitive function. Over months, brain changes begin to normalize.
Other options include:
- Oral appliances: A custom mouthguard that advances your jaw forward, opening your airway
- Position therapy: Sleeping on your side instead of your back (lateral positioning devices exist for this)
- Surgical options: Procedures to expand the airway (UPPP, LAUP) for selected patients
- Weight loss: If obese, weight loss can significantly improve or even resolve sleep apnea
The key is identifying sleep apnea and treating it. Untreated, it's one of the strongest drivers of brain aging.
Chronotype Alignment
If your work schedule conflicts with your natural chronotype, addressing this can have major benefits. Options:
- Shift work: If possible, switch to a work schedule that matches your natural rhythm
- Flexibility: Negotiate flexibility with your employer to start work later or earlier
- Light therapy: Strategic light exposure can shift your chronotype somewhat (typically 30-90 minutes of bright light at specific times)
- Melatonin timing: Taking melatonin several hours before your desired sleep time can shift your rhythm forward, though it's less effective shifting it backward
The goal isn't to change your personality. Early risers shouldn't feel pressure to become night owls. It's about reducing the mismatch between your natural rhythm and your schedule demands.
Sleep Duration Optimization
The research suggests 7-8 hours is optimal for brain health. But the key is consistent duration. Your brain prefers consistency.
Sleeping 6 hours every night might be better than sleeping 6 hours some nights and 9 hours other nights. This is because sleep-wake cycles regulate your circadian rhythm. Consistency strengthens the rhythm.
Optimizing duration means:
- Consistency: Same bedtime and wake time every day (including weekends)
- Sufficiency: Ensuring you're getting toward the 7-8 hour range
- Not forcing: If you naturally need 6.5 or 9 hours, honoring that rather than forcing yourself into 8
Medication Considerations
For people with insomnia who don't respond to behavioral approaches, medication can help. Options include:
Non-Benzodiazepine Hypnotics: Medications like zolpidem, zaleplon, eszopiclone. They're shorter-acting than benzodiazepines and have less next-day hangover effect. Effective but tolerance can develop.
Benzodiazepines: Longer-acting, more addictive, more next-day impairment, but effective for acute insomnia. Generally avoided for chronic insomnia due to dependency risk.
Melatonin Receptor Agonists: Ramelteon acts on melatonin receptors, promoting sleep without the addictive properties of traditional sedatives.
Orexin Receptor Antagonists: Suvorexant blocks wake-promoting signals, allowing sleep. Newer option with different mechanism than traditional sedatives.
Tricyclic Antidepressants: Low-dose amitriptyline or doxepin sometimes used for insomnia, especially when anxiety is a component.
SSRI Antidepressants: Paroxetine and other SSRIs sometimes used for insomnia when depression or anxiety is present.
The key principle: medication works best as an adjunct to behavioral changes, not a replacement for them. Using a medication while also implementing sleep hygiene and behavioral changes produces better outcomes than medication alone.

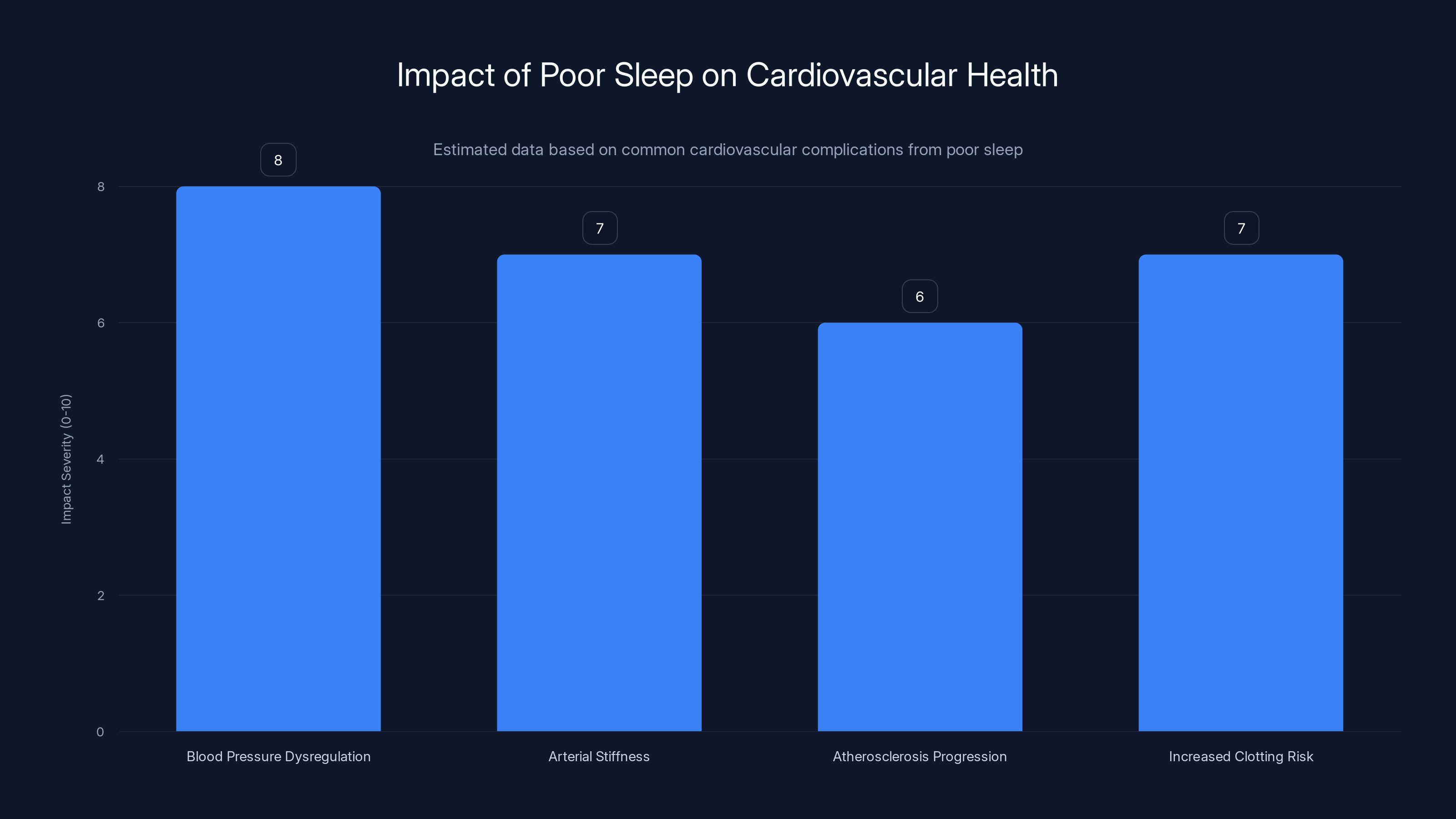
Poor sleep significantly impacts cardiovascular health, with blood pressure dysregulation and increased clotting risk being the most severe complications. Estimated data.
Practical Sleep Optimization: 30-Day Sleep Protocol
Here's a specific, actionable approach to optimizing sleep quality based on the research:
Week 1: Assessment and Sleep Hygiene
- Track your sleep: Use a simple sleep diary or app to record: bedtime, wake time, perceived sleep quality (1-10 scale), nighttime awakenings, morning alertness
- Optimize your bedroom: Temperature 65-68°F, completely dark (blackout curtains), quiet (earplugs if necessary), clean and decluttered
- Establish consistent times: Same bedtime and wake time, even weekends (within an hour)
- Limit afternoon caffeine: No caffeine after 2-3 PM
- Avoid alcohol before bed: Alcohol fragments sleep and reduces deep sleep
Week 2: Assess Sleep Debt and Light Exposure
- Calculate sleep need: Track your natural sleep duration if you sleep without an alarm over a weekend
- Morning light exposure: Get 15-30 minutes of bright light exposure within 30 minutes of waking
- Afternoon light avoidance: Avoid bright light after 3-4 PM
- Evening routine: Begin winding down an hour before bed; dim lights, avoid screens
- Monitor caffeine: Ensure no caffeine consumption after 2 PM
Week 3: Sleep Consolidation
- Restrict time in bed: If you're spending 9 hours in bed but sleeping 7, spend only 7-7.5 hours in bed initially. This consolidates sleep and reduces waking time in bed
- Bed boundary: Use your bed only for sleep and sex. Work, eating, watching TV all happen elsewhere
- Stimulus control: If you're awake in bed for more than 20 minutes, get up and do something boring and dimly lit until you feel sleepy
- Wind-down ritual: Consistent 30-minute routine before bed (reading, journaling, stretching)
Week 4: Refinement and Troubleshooting
- Assess progress: Review your sleep diary. What's improved? What's still problematic?
- Adjust sleep duration: If sleeping well, gradually increase time in bed by 15-30 minutes until you find your optimal duration
- Address remaining issues: Identify specific problems (racing thoughts, partner disturbance, physical discomfort) and target interventions
- Consider professional help: If significant insomnia remains, consult a sleep medicine specialist
This four-week protocol addresses the modifiable behaviors driving poor sleep. For some people, these changes produce dramatic improvements. For others, underlying sleep disorders or mood disorders require professional treatment.
Emerging Research: Sleep and Neurodegenerative Disease
Beyond brain aging, emerging research connects poor sleep to specific neurodegenerative diseases.
Beta-amyloid accumulation, which the glymphatic system clears during sleep, is the hallmark of Alzheimer's disease. Tau protein tangles, similarly cleared during sleep, are also prominent in Alzheimer's and other tauopathies. Poor sleep accelerates accumulation of both.
Studies tracking people over years show that poor sleep quality predicts faster cognitive decline and earlier dementia onset. People sleeping poorly at baseline are at 2-3x higher risk for developing Alzheimer's within 10-15 years.
For Parkinson's disease, REM sleep behavior disorder (where people physically act out dreams) often precedes motor symptoms by years. Sleep disruption is common in Parkinson's and might contribute to disease progression.
For Lewy body dementia, sleep disorders are extremely common and might contribute to pathology.
The implication: sleep quality is not just a cosmetic health factor. It's a critical preventative factor for dementia risk. Optimizing sleep in your 50s and 60s likely reduces your dementia risk in your 80s.
What We Still Don't Know: Open Questions in Sleep and Brain Aging
While the research is compelling, important questions remain unanswered.
Is brain aging reversible? The research shows correlations between poor sleep and brain aging, but doesn't conclusively prove that improving sleep reverses the aging. Do people who improve their sleep see their biological brain age decrease? Research is ongoing.
Individual variation: Why do some people tolerate poor sleep better than others? Genetic variation in clock genes, inflammatory response genes, and neuroprotective mechanisms probably matters, but we don't fully understand the genetic basis yet.
Optimal sleep duration: The 7-8 hour recommendation works for most people, but is it truly optimal? Does it vary by age? By genetics? By lifestyle? We're still refining this.
Sex differences: The research didn't prominently break down findings by sex, but emerging evidence suggests women might be more vulnerable to sleep deprivation's effects on brain health. Why?
Critical periods: Is there a window during which sleep optimization is most important? Is middle age when you start optimizing sleep most important for later dementia prevention?
Interventions: Beyond the behavioral approaches discussed, what other interventions could help? Supplements? Pharmacological approaches? Brain stimulation techniques?
These questions represent the frontier of sleep neuroscience. Over the next decade, answers to these questions will likely refine our understanding and recommendations.
The Sleep Argument: Why This Matters to You Personally
Let's connect this back to your life.
You have a finite amount of brain aging ahead of you. It's not unlimited. You lose about 0.5% of your brain volume per year after age 30. That's inevitable. But you can slow it down or speed it up based on your choices.
Poor sleep speeds it up. Good sleep slows it down. That's not metaphorical. That's literally what the brain scans show.
The difference between someone who sleeps well and someone who doesn't could be equivalent to 5-10 extra years of brain aging over a decade. In your 60s or 70s, 5-10 years of cognitive decline is the difference between independence and dependence. Between recognizing your grandchildren and not.
And here's the beautiful part: unlike most health problems, sleep is something you can directly control. You can't change your genes. You can't rewind past damage. But you can change your sleep starting tonight.
The protocol outlined above isn't revolutionary. It's proven, evidence-based approaches that neuroscientists recommend. It takes effort. It requires changing habits. It might involve uncomfortable conversations with your employer about scheduling flexibility or with your doctor about treating sleep apnea.
But the payoff is concrete: a brain that ages more slowly, that functions better, that carries you into old age with your cognition intact.
That's worth a bit of effort.

FAQ
How exactly does poor sleep age the brain?
Poor sleep accelerates brain aging through multiple pathways: chronic inflammation damages brain tissue, the glymphatic system fails to clear toxic proteins like beta-amyloid, and cardiovascular dysfunction reduces oxygen delivery to brain regions. These mechanisms combine to make the brain biologically older than its chronological age, which is measurable on MRI brain scans using machine learning models trained to detect age-related changes.
Can brain aging from poor sleep be reversed?
Partial reversal appears possible. While the research doesn't conclusively prove that improving sleep reverses biological brain age, studies show that when people treat sleep disorders like sleep apnea or improve their sleep habits, brain structure and function begin to normalize. The earlier you address poor sleep, the better the potential for reversal.
How much sleep do I actually need?
The research suggests 7-8 hours is optimal for most people, but individual needs vary based on genetics. The key is finding your personal optimal duration (which might be 6.5 or 9 hours) and maintaining consistency. What matters more than the exact number is that you sleep the same duration consistently and wake feeling refreshed.
If I'm a night owl, does that mean I'm predestined to brain aging?
No. Being a night owl isn't inherently problematic. The issue is chronotype mismatch—being a night owl forced into a morning schedule. If you can work a schedule aligned with your natural rhythm, you avoid the brain aging acceleration. If you can't, the sleep quality and quantity matter even more.
How do I know if I have sleep apnea?
Common signs include snoring, gasping for air during sleep, daytime sleepiness despite adequate sleep duration, morning headaches, and non-restorative sleep. If you experience these symptoms, ask your doctor for a sleep study. Sleep apnea is common, often undiagnosed, and highly treatable.
What's the fastest way to improve sleep quality?
For most people: establish consistent sleep-wake times (same every day), optimize your bedroom (cool, dark, quiet), and address any underlying sleep disorders. These behavioral changes work as well as medication for most insomnia and don't carry side effects. If behavioral changes don't work within 2-3 weeks, consult a sleep medicine specialist.
Does melatonin help with brain aging prevention?
Melatonin is an antioxidant and has some neuroprotective properties. For some people with circadian rhythm disorders, melatonin at the right time can improve sleep quality. However, for sleep quality optimization, establishing consistent sleep-wake times and sleep hygiene are typically more effective than melatonin supplementation.
How long does it take to see improvements from optimizing sleep?
Some improvements appear within days (more alertness, better mood), but brain structure changes take longer. Brain imaging changes appear within weeks to months of sustained sleep improvement. The full neuroprotective benefit likely accumulates over months and years of consistently good sleep.
Is sleeping more than 9-10 hours per night harmful?
Consistently sleeping significantly more than 9-10 hours is associated with increased mortality and increased brain aging risk in research. This usually indicates an underlying problem—sleep apnea causing fragmented sleep, depression, or medical illness. If you find yourself sleeping 10+ hours and still exhausted, that's worth investigating with a doctor.
Can I catch up on sleep debt from the week with weekend sleep?
Partially. If you sleep poorly Monday-Friday and then sleep 10 hours Saturday-Sunday, you recover some of the acute tiredness. But you don't fully recover the neurological benefits of consistent good sleep throughout the week. The research emphasizes that consistency matters as much as total quantity. Erratic sleep schedules are associated with worse outcomes than consistent sleep, even if the total is the same.
The Long Game: Sleep as Brain Insurance
Here's a thought experiment. Imagine two people. Both are 55 years old. Both have the same genes, the same income, the same lifestyle. The only difference: one sleeps 7-8 hours nightly with good quality. The other sleeps 5-6 hours with frequent awakenings.
Based on the research, by age 65, the second person's brain is biologically equivalent to someone in their early 70s. They're experiencing cognitive changes—processing slower, memory slightly worse, word-finding harder—that seem normal for their age but are actually 5-10 years accelerated.
By age 75, the accumulation is even more dramatic. The good sleeper still has most of their cognitive function. The poor sleeper is struggling, might be showing early signs of dementia, is requiring more help with complex tasks.
The difference compounds. A disease that might have onset at 80 in the good sleeper appears at 75 in the poor sleeper. Independence is lost earlier. Quality of life is diminished sooner.
This isn't inevitable. It's a direct consequence of choices made year after year.
The research in this article gives you the knowledge. The sleep optimization protocol gives you the tool. What remains is the decision.
Sleep well. Your brain tomorrow depends on it.

Key Takeaways
- Poor sleep quality makes your brain biologically approximately 1 year older than your actual age, measured via MRI and machine learning models
- Each point decrease in sleep quality equals roughly 6 months of accelerated brain aging across multiple dimensions (chronotype, duration, insomnia, snoring, daytime sleepiness)
- Chronic inflammation explains 7-10% of the sleep-to-brain-aging association, while glymphatic system failure to clear toxic proteins is a major additional mechanism
- Night owl chronotype, unhealthy sleep duration (beyond 7-8 hours), and snoring are the strongest individual predictors of brain aging
- Sleep quality is highly modifiable through behavioral approaches (CBT-I, chronotype alignment, sleep hygiene) and treatment of underlying disorders (sleep apnea, insomnia)
- Brain aging from poor sleep appears partially reversible when sleep quality improves, making sleep optimization a powerful preventative health intervention
![How Poor Sleep Accelerates Brain Aging: Science & Prevention [2025]](https://tryrunable.com/blog/how-poor-sleep-accelerates-brain-aging-science-prevention-20/image-1-1767184733944.jpg)



