The Future of Period Tech: How a Smart Pad Became a Hormone Laboratory
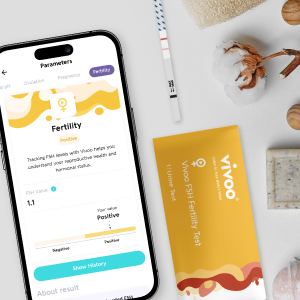
Your period is about to tell you something important about your health. Not just that you're menstruating, but whether your ovarian reserves are declining, if you might be heading toward perimenopause, or whether you're dealing with hormonal conditions like PCOS. That's the promise of the Vivoo Flow Pad, a menstrual pad that's essentially a portable hormone test tucked into a product billions of people use every single day.
When Vivoo CEO Miray Tayfun first pitched the idea, it sounded almost too simple. A menstrual pad that measures your follicle-stimulating hormone (FSH) levels without requiring a blood draw, a lab visit, or even leaving your bathroom. You bleed into the pad like you normally would, and when you're ready to change it, you get instant results on the back of the pad itself. No equipment. No waiting for results from a lab. Just biology doing its job while providing data you've never had access to before.
This isn't hype. This is actually happening. At CES 2026, Vivoo revealed a product that bridges the gap between hygiene and health technology in a way that feels almost obvious in retrospect but required significant biomedical engineering to make real. The Flow Pad represents a fundamental shift in how we think about period tech, at-home diagnostics, and women's health data collection.
Here's what you need to know: the pad costs roughly
But before you assume this is a miracle product, let's dig into what it actually does, how it works, why it matters, and what the real limitations are.
TL; DR
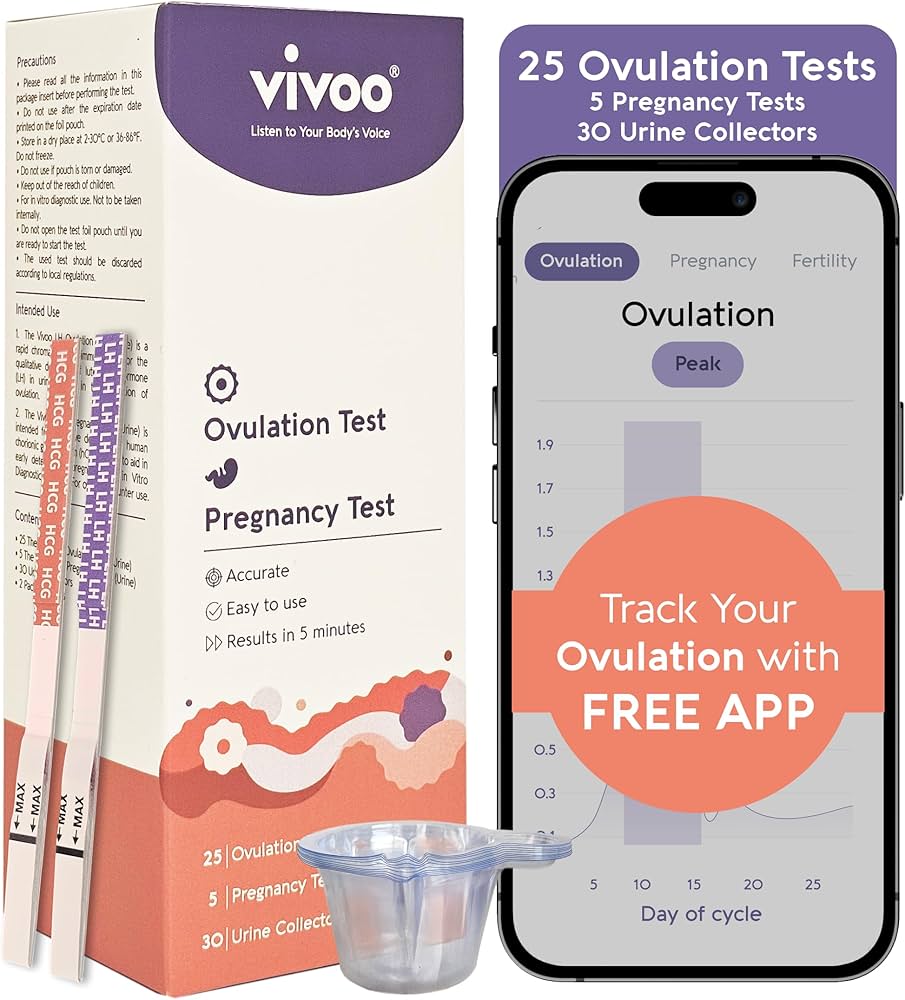
- What it is: A menstrual pad with embedded microfluidic channels that measure FSH hormone levels in menstrual blood
- How much it costs: 5 per pad, with potential subscription and pack pricing coming at launch
- Target users: People aged 30–45 interested in fertility tracking, hormonal condition management, or perimenopause monitoring
- Main advantage: Combines a product people already use with a diagnostic test that normally requires a doctor's office visit
- Launch timeline: Currently in early access for researchers and medical partners; broader rollout timeline not yet announced
- Bottom line: This is early-stage tech that could meaningfully expand access to hormone testing, though it's not a replacement for comprehensive medical evaluation

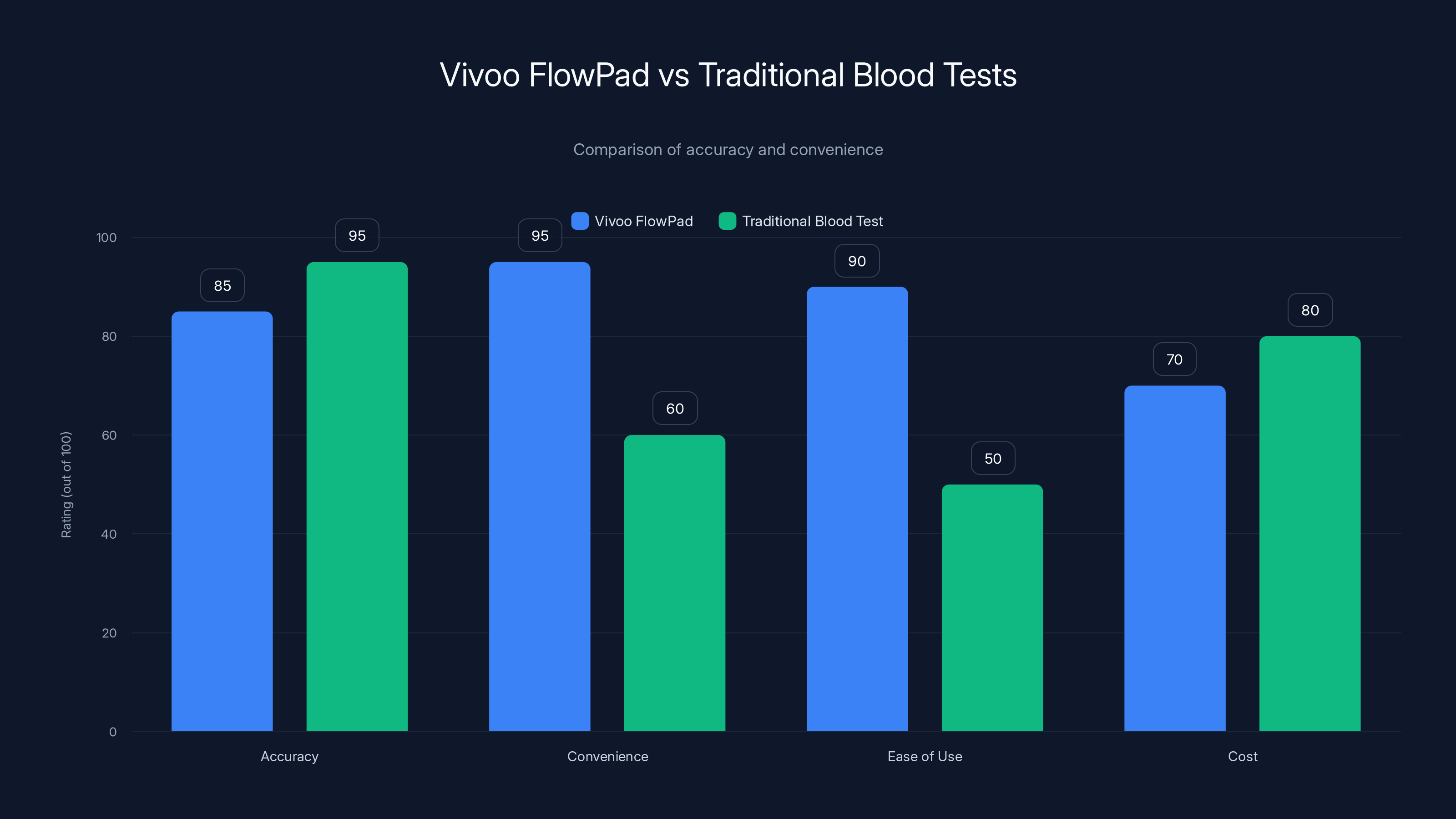
The Vivoo FlowPad offers higher convenience and ease of use compared to traditional blood tests, although traditional tests are slightly more accurate. Estimated data.
Understanding FSH and Why Your Menstrual Pad Now Tests For It
Before we talk about how the Flow Pad works, you need to understand what FSH actually is and why measuring it matters.
Follicle-stimulating hormone is produced by your pituitary gland and plays a central role in your reproductive system. During each menstrual cycle, FSH levels rise and fall in a predictable pattern. This hormone tells your ovaries to start developing follicles—little sacs containing eggs. When FSH does its job correctly, you get regular ovulation and menstrual cycles. When something goes wrong—whether that's aging ovaries, hormonal conditions, or approaching menopause—FSH levels change in detectable ways.
Traditionally, measuring FSH required scheduling a doctor's appointment, having blood drawn, waiting for lab results, and paying somewhere between
High FSH levels can indicate several things, and understanding what they mean requires some context. If you're in your fertile years and your FSH is elevated, it might suggest diminished ovarian reserve—basically, your ovaries have fewer eggs than they should. That matters if you're trying to conceive because it affects your fertility window and odds of successful pregnancy. FSH elevation can also be a sign of conditions like polycystic ovary syndrome (PCOS), where hormonal imbalances affect both fertility and overall health. And if you're moving through your 40s, rising FSH often signals the approach of perimenopause, the transition phase before menopause that can last five to ten years and come with its own set of symptoms and health considerations.
The brilliance of testing FSH through menstrual blood—rather than serum blood like traditional tests—is both practical and scientific. You're directly testing the biological material your body is already shedding. You don't need a needle. You don't need to schedule anything. The test happens as a natural part of your cycle.
But here's the caveat: a single FSH reading tells you something, but not everything. FSH levels fluctuate significantly throughout your cycle and can vary from cycle to cycle. A truly comprehensive fertility workup typically includes multiple FSH tests, imaging to assess ovarian reserve, testing other hormones like estradiol, and potentially genetic or other diagnostic markers. The Flow Pad gives you one valuable data point, not a complete picture. That's important to understand before interpreting results.

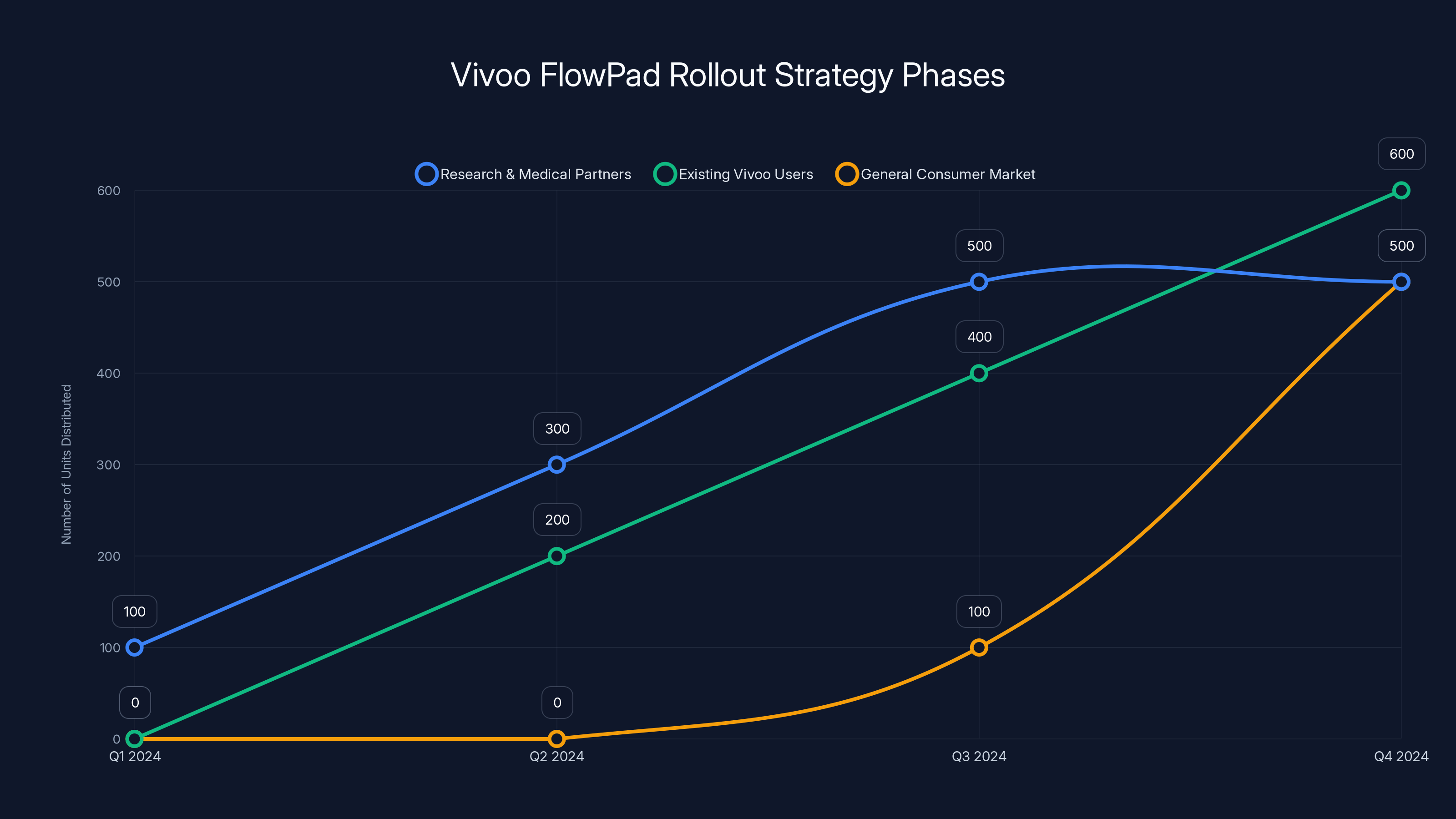
Vivoo's phased rollout strategy begins with researchers and medical partners, followed by existing users, and finally the general market. Estimated data shows a gradual increase in distribution.
The Biomedical Engineering Behind a Pad That Thinks
Building a hormone test into a menstrual pad sounds conceptually straightforward until you actually try to do it. Then the engineering challenges become apparent, and Vivoo's approach to solving them is genuinely clever.
The core problem: blood dries quickly, and dried blood doesn't react with the chemical reagents needed to detect hormones. In traditional at-home hormone tests—the kind you buy at a pharmacy—users have to actively dilute their blood sample with a solution provided in the kit. You're controlling the chemistry. You're following instructions. You have time and space to perform steps. None of that works when the test is embedded in an absorbent pad that you're actively bleeding into.
Vivoo had to replicate the chemistry that happens in those traditional tests but build it directly into the pad structure itself. They did this by creating what they call a "capillary capture layer" as the top functional component of the pad.
Here's what's happening beneath the surface: that top layer draws in menstrual blood and simultaneously filters out particulates—cell debris, fibrin, other materials that aren't useful for hormone detection. It's like a microscopic sieve that separates the useful biological material from the rest. Think of it like making coffee: you want the flavorful liquid through, but you need the filter to catch the grounds.
Below that capillary layer sits what Vivoo calls the "biomarker reaction layer." This layer contains stabilized chemical reagents—basically, pre-loaded substances that are designed to interact with FSH molecules specifically. When the filtered menstrual blood meets these reagents, a chemical reaction occurs. That reaction produces a visible result in the windowed area on the back of the pad.
The result looks like a pregnancy test or COVID-19 test: you get a line or color indication. The intensity or presence of the line correlates to your FSH level. It's simple enough that anyone can interpret it without special equipment, yet sophisticated enough that it's measuring an actual hormone in real time.
One of the most technically challenging aspects of this design is maintaining the chemical stability of those reagents while they're sitting in an absorbent pad waiting to come into contact with blood. Reagents can degrade, become less effective, or lose their ability to detect target hormones if they're exposed to moisture, temperature changes, or light. Vivoo had to engineer protective measures into the pad structure itself—essentially creating a chemically stable environment for reagents while still allowing blood to flow through and activate them.
There's also the matter of reducing false positives and false negatives. A hormone test is only valuable if it's accurate. Vivoo would have needed to validate their method against traditional laboratory testing to ensure that FSH readings from their pad correlate reliably with serum FSH levels from blood draws.
The pad geometry itself matters too. Pads vary in absorbency, material composition, and shape. Vivoo had to design the microfluidic channels—the tiny channels that guide blood flow through the reactive layers—to work reliably regardless of normal variations in pad positioning or the amount of blood present on any given day of a person's cycle.
From Vaginal p H Liner to Hormone-Measuring Pad: Vivoo's Evolution
The Flow Pad didn't emerge fully formed from Vivoo's labs. The company has been iterating on period tech for several years, and understanding that evolution provides important context for what they've built.
Vivoo started with a different product: a vaginal p H tracking liner. If that sounds niche, well, it was. The idea was that vaginal p H changes throughout your cycle and in response to infections or other health issues. A color-changing liner would give users real-time feedback about their vaginal health. It's actually a useful concept—vaginal p H dysbiosis can indicate bacterial vaginosis, yeast infections, or hormonal imbalances.
But color-changing liners are limited. They give you qualitative data (your p H seems off) rather than quantitative data (your p H is exactly 4.2). They're primarily reactive, not predictive. And frankly, while vaginal health is important, most people don't check their p H status as a priority health metric.
Vivoo listened to user feedback and market signals, and they evolved. They moved from p H tracking to something more systemically valuable: hormonal tracking through the one fluid everyone who menstruates produces on a predictable schedule. That's menstrual blood. And the delivery mechanism evolved from a liner to a pad—a product that's more mainstream and less stigmatized as a wellness gadget.
That journey from p H liner to hormone-testing pad tells you something important about biotech companies and product development. The initial idea might not be the final product. But each iteration gets you closer to something genuinely useful. Vivoo's pivot wasn't a failure of the original concept; it was a realistic appraisal of what users actually wanted and what would drive adoption.
The company is also being thoughtful about their business model from the start. They're not positioning the Flow Pad as a "forever product" that becomes essential infrastructure like traditional pads. Instead, they're envisioning it as a tool you use when it's useful—maybe for a few months if you're trying to conceive, or intermittently if you're tracking hormonal patterns over time. That's a sustainable messaging approach because it's honest. Not everyone needs hormone tracking every single period. But many people would benefit from accessing that data when it matters.
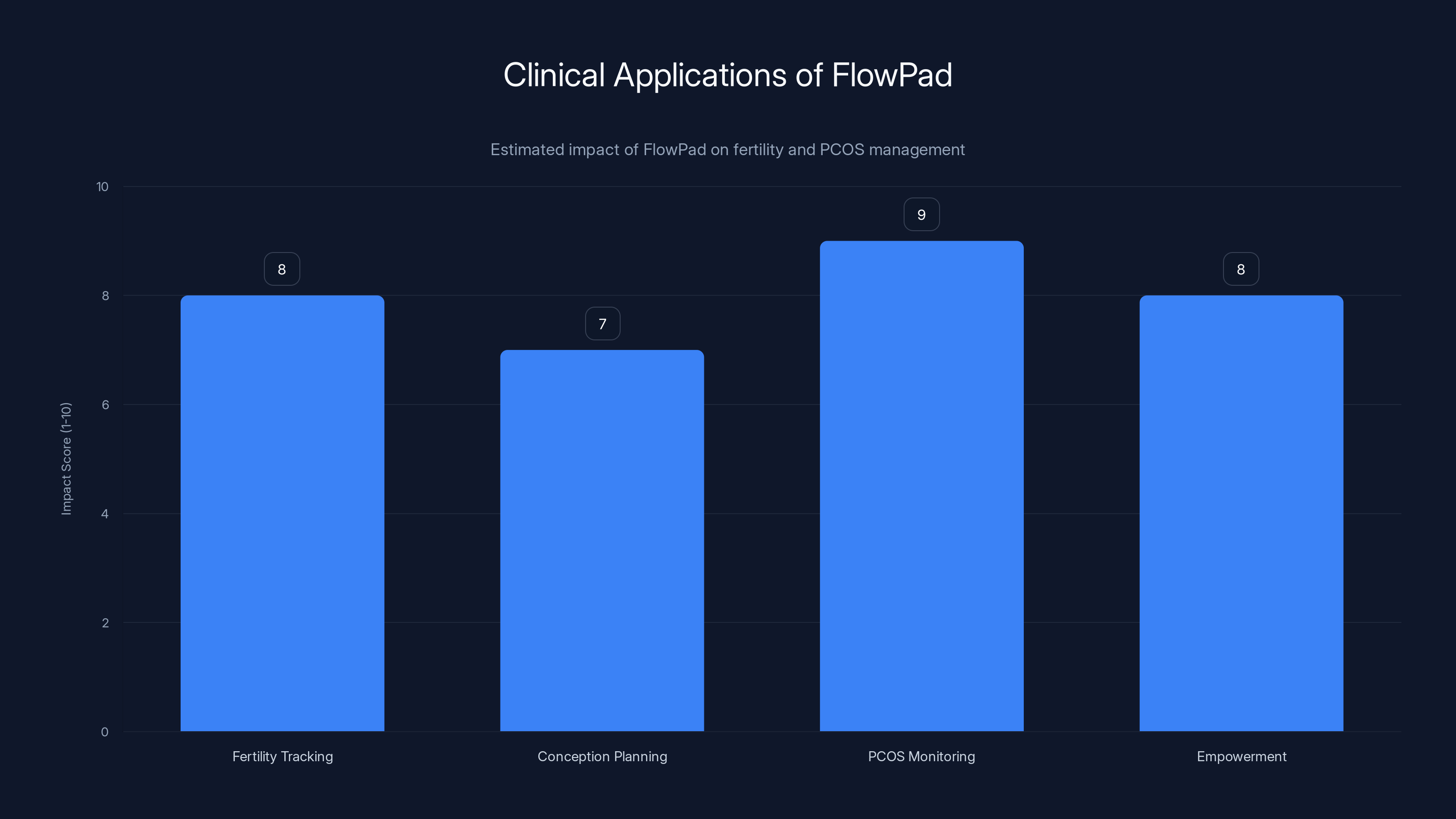
FlowPad significantly impacts fertility tracking, conception planning, and PCOS management by providing accessible, self-generated health data. Estimated data.
The Clinical Applications That Make This More Than a Gadget
Why does this technology matter beyond the "cool factor" of a smart pad? Because it addresses real gaps in healthcare access and reproductive health monitoring.
Fertility Tracking and Conception Planning
For people trying to conceive, knowing your ovarian reserve status is valuable information. FSH levels are a standard marker that fertility specialists use to assess egg quantity and quality. Historically, accessing this information meant scheduling a consultation with a fertility specialist and getting bloodwork done. That requires time, money, and often involves insurance navigation or out-of-pocket costs.
The Flow Pad makes this information more accessible. Someone could use it to track their FSH over several cycles before deciding whether to pursue fertility treatment, to get baseline data before paying for a specialist consultation, or to monitor whether their FSH changes over time. For people in their late 30s and early 40s—prime years for fertility concerns but not yet in crisis mode—this kind of accessible testing could genuinely help with family planning decisions.
There's a secondary benefit here around empowerment. Traditionally, your fertility status was something your doctor tells you about. You go in, get tested, get results, and then you process them with a healthcare provider. With the Flow Pad, you're generating that data yourself as part of your normal routine. That shift from passive recipient to active participant in understanding your own health is psychologically significant.
PCOS Monitoring and Management
Polycystic ovary syndrome affects roughly 6-20% of menstruating people globally. It's a condition with no cure, but it's highly manageable with proper monitoring and treatment. PCOS involves hormonal imbalances—elevated testosterone, irregular FSH patterns, insulin resistance—that require ongoing tracking.
People with PCOS often need regular hormone testing to monitor their condition, assess whether treatment is working, and catch complications early. That means regular doctor's visits and blood tests. The Flow Pad could significantly reduce the number of office visits required, allowing people to do basic monitoring at home and only schedule in-person visits when results indicate they need adjustment or intervention.
For someone managing PCOS, being able to track FSH trends over three, six, or twelve months without needing twelve separate lab visits is genuinely valuable. It's cheaper, less time-intensive, and reduces healthcare friction for a chronic condition that requires ongoing attention.
Perimenopause Tracking
Perimenopause is weird. Your period doesn't stop; it gets irregular. Your hormones fluctuate wildly. You might experience hot flashes, mood changes, sleep disruption, joint pain, and a constellation of other symptoms that vary day to day. And nobody tells you this ahead of time. Many people experience symptoms for years before realizing they're in perimenopause.
FSH testing is one of the standard ways to assess perimenopause status. Rising FSH indicates your ovaries are producing less estrogen, signaling the approach of menopause. Multiple testing points over time show the pattern and help gauge how far into perimenopause someone is.
The Flow Pad could transform perimenopause management from a guessing game into something data-informed. Someone experiencing symptoms could use the pad to track whether their FSH is actually rising (indicating perimenopause) or whether something else might be going on. They could show data to their healthcare provider. They could monitor how perimenopause progresses over time. For people in their 40s and early 50s, this is huge.
The Rollout Strategy: Early Access, Research Partnerships, and Staged Launch
Vivoo isn't just launching the Flow Pad to the general public immediately. They're being strategic about how they introduce it to the market, and that strategy reveals something about how they're thinking about validation, adoption, and scaling.
The initial rollout is happening in phases. First come researchers and medical partners. This serves multiple purposes. Researchers can validate the technology in peer-reviewed studies. Medical professionals can understand how to explain results to patients. Real-world data collection happens in a controlled way. And Vivoo gets evidence of efficacy that strengthens their position with regulators and healthcare providers.
Second phase involves existing Vivoo users. People who've already bought their previous products (like the p H tracking liner) have shown interest in this category and trust the brand. They're a ready audience who can provide feedback, testimonials, and word-of-mouth marketing.
Only after those phases would broader consumer availability happen. That's actually a smart approach for a biotech product that makes health claims. You want initial validation. You want early adopters who understand the product's strengths and limitations. You want physician awareness and buy-in. You want to understand real-world usage patterns. You want media coverage and social proof. All of that happens through phased rollout more effectively than day-one mass market launch.
The pricing strategy also reveals confidence in the product. At
Subscription and pack configurations are coming, which suggests they're thinking about recurring revenue and customer lifetime value. Someone might buy a monthly subscription of pads designed for hormone tracking, or they might buy multi-packs at a discount. That's standard business model thinking, but it also indicates belief in long-term demand.

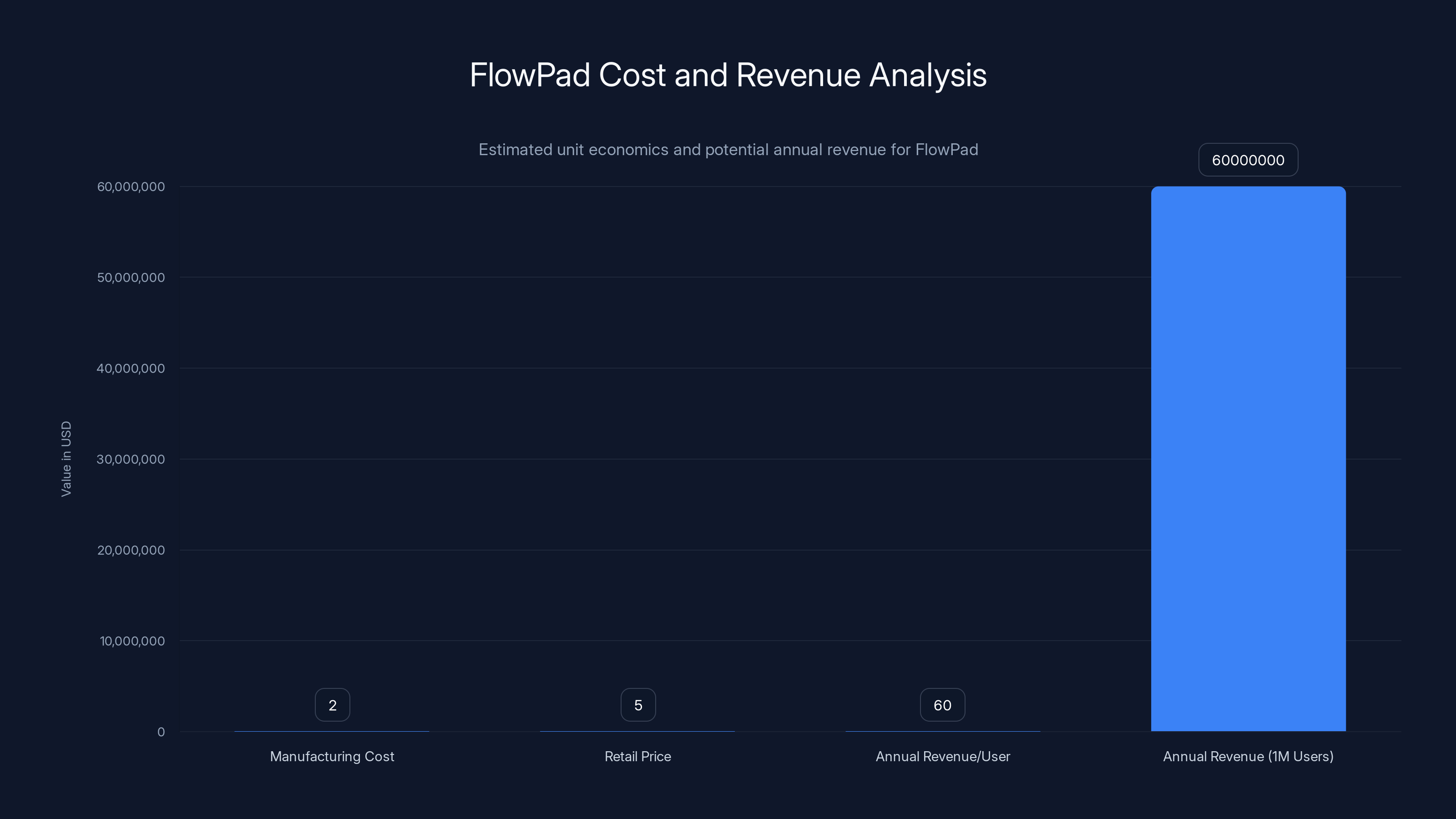
Estimated data shows FlowPad's manufacturing cost at
What the Research Base Actually Shows About At-Home Hormone Testing
Before you assume this will revolutionize how you understand your body, let's talk about what at-home hormone testing actually reveals and what limitations remain.
At-home hormone tests have exploded in the wellness market over the past five years. You can buy tests for progesterone, testosterone, estrogen, cortisol, and more, usually ordered online, completed at home, and analyzed by a company that sends back a report. The appeal is obvious: convenience, privacy, ability to test multiple times.
But here's the complexity: a single hormone test tells you something, but that something requires context to be meaningful. FSH fluctuates throughout your cycle by design. Your FSH on day 3 will be different from your FSH on day 21. Estrogen and progesterone rise and fall in coordinated patterns. Testosterone varies based on time of day, time of cycle, stress level, sleep, exercise, and other factors. Testing without understanding this context can lead to misinterpretation.
That's why medical doctors typically want to see multiple data points over time, taken at consistent points in the cycle, ideally with other hormones tested simultaneously for comparison. A single FSH reading from a Flow Pad is useful information. It's not a diagnosis. It's not enough to confirm or rule out most conditions on its own. It's a data point that becomes more meaningful when combined with other information—your symptoms, your cycle patterns, your medical history, other test results.
The research on at-home hormone testing also shows wide variability in accuracy depending on the test, the company, and the hormone being measured. Some at-home tests have been validated against traditional lab testing. Others haven't. Some are more accurate than others. The fact that Vivoo is planning to work with research partners to validate their technology is encouraging, but we don't yet know how the Flow Pad's accuracy compares to traditional FSH blood tests.
Another consideration: interpretation of results. Traditional lab tests come with reference ranges that have been developed through population studies. Your FSH result is compared against that range, and your doctor tells you what it means. At-home tests often don't come with that context, or they come with app-based analysis that's only as good as the algorithm behind it. An elevated FSH reading is meaningful, but elevated relative to what baseline? Different labs use different reference ranges. Your result could be "abnormal" by one lab's standards but "normal" by another's.
The Flow Pad's integrated app will likely address some of this by providing interpretation and context. But that interpretation is only as good as the algorithm and the data it's been trained on. And we'll want to see research showing that the app's interpretations align with what a reproductive endocrinologist would tell you.
Comparison to Other Period Tech and Reproductive Health Gadgets
The Flow Pad isn't the first product trying to make menstrual cycles more transparent and measurable. Understanding how it compares to other innovations in this space helps contextualize what makes it unique.
Smart Menstrual Cups
Companies like Emm have developed smart menstrual cups with biosensors embedded to track flow volume, duration, and biomarkers. The advantage of a smart cup is that it's reusable and collects detailed data over time. The disadvantage is the barrier to adoption—not everyone uses menstrual cups, and switching to one is a bigger behavior change than the Flow Pad requires. Also, smart cups focus on flow and menstrual pattern rather than hormones, so they answer different questions.
Wearable Fertility Trackers
Devices like Oura Ring, Whoop, and other wearables claim to track fertility through biometrics like temperature, heart rate variability, and sleep. These give you trend data and can potentially flag when you're in your fertile window based on temperature rises. But they're inference-based—they're predicting fertility from indirect signals rather than measuring the hormones that actually control ovulation. Accurate but not direct.
Period Tracking Apps
Apps like Flo, Clue, and others use calendar-based prediction, symptom tracking, and optional integration with other data to help people understand their cycles. They're useful for pattern recognition but don't include any biological measurement. You're relying on accurate self-reporting and the app's algorithm accuracy.
The Flow Pad is different because it's the first product I'm aware of that combines menstrual product functionality with direct hormone measurement without requiring separate equipment, blood draws, or lab visits. That combination of simplicity, directness, and integration into an existing product people already use is genuinely novel.

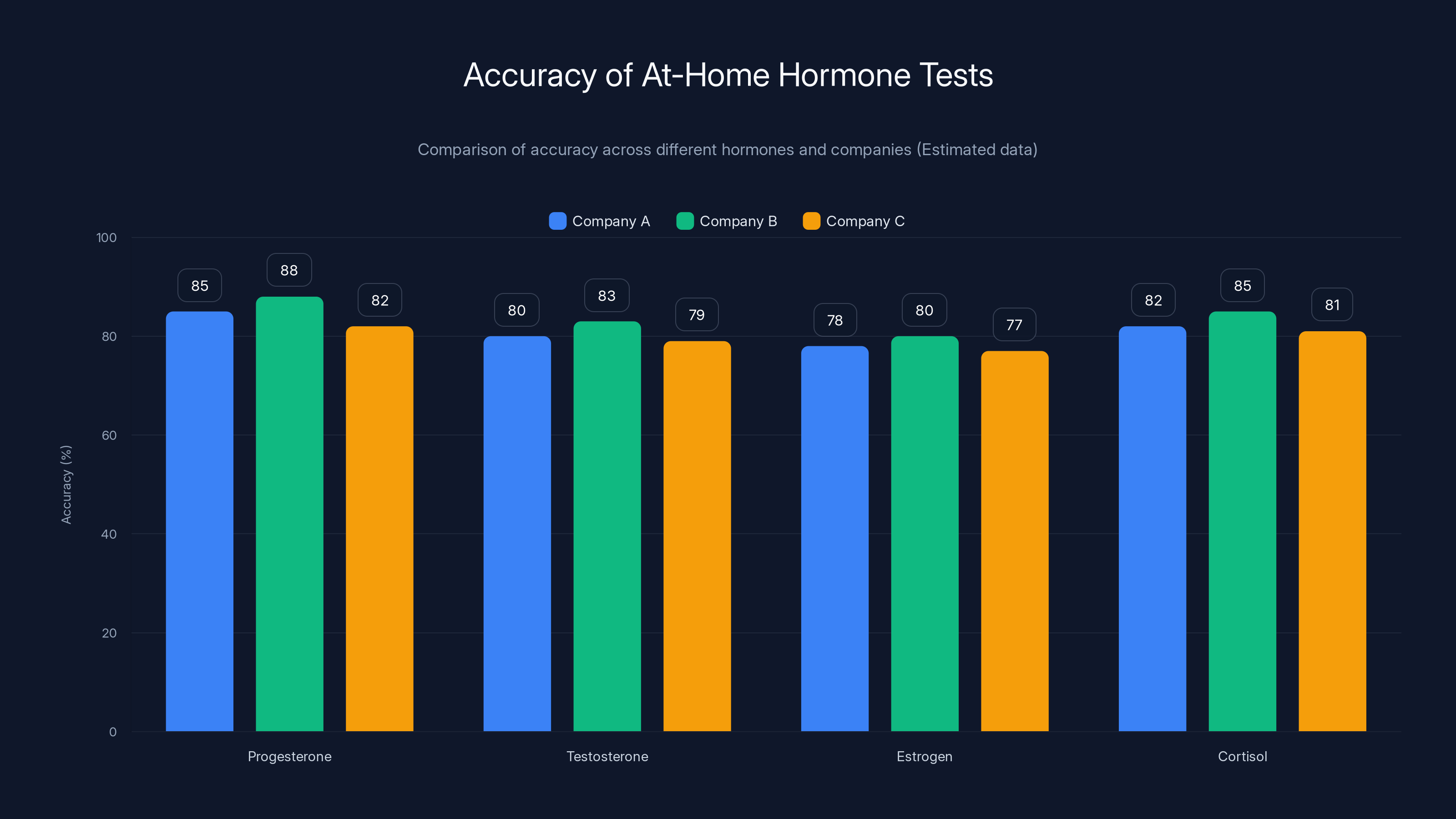
Estimated data shows variability in accuracy of at-home hormone tests across different companies and hormones. Progesterone and cortisol tests tend to have higher accuracy compared to testosterone and estrogen.
The Privacy and Data Security Considerations You Should Know About
Any product that collects health data raises privacy questions. The Flow Pad is no exception. You'll be generating sensitive reproductive health data, and that data will be transmitted to Vivoo's servers for analysis.
The obvious questions: Who has access to your data? How is it stored? How long is it kept? Could it be sold? Could it be breached? Could law enforcement or other entities access it? These aren't paranoid questions—they're standard due diligence for any health data product.
Vivoo hasn't published detailed privacy documentation yet because the product is still in early access. But these are questions worth asking before you decide to use the Flow Pad. Look for clear privacy policies, data minimization practices, encrypted transmission and storage, and explicit policies about third-party access.
There's also the question of data permanence. Once you generate health data about your hormonal status, you can't really un-generate it. That data exists. Years later, could it be used in ways you didn't anticipate? Could it affect insurance rates, employment decisions, or other aspects of your life? That's science fiction in most cases, but it's science fact in some contexts. Be aware that reproductive health data is particularly sensitive and potentially subject to scrutiny depending on where you live and how laws around reproductive health evolve.
For people in jurisdictions where reproductive autonomy is under legal attack, sharing detailed hormone data might feel like an unnecessary risk. That's a legitimate concern. The flip side is that if you're trying to get pregnant or manage a condition, that same data is incredibly valuable. It's a tradeoff that each person needs to evaluate for themselves.
Vivoo should absolutely publish transparent privacy policies, security documentation, and data handling practices. If they haven't when the product launches, that would be a red flag. You're trusting them with health data. That trust should be backed by documented security practices and clear policies.

The Regulatory Landscape: What It Takes to Sell a Hormone Test
Here's something people don't always think about: the Flow Pad makes medical claims. It measures a hormone. It's diagnosing something. That puts it squarely in the regulatory domain.
In the United States, the FDA regulates medical devices. A product that measures a biomarker and provides results is a medical device. Depending on how Vivoo markets it and how sophisticated it is, it might need FDA clearance before it can be sold to consumers. The good news: at-home diagnostic tests have cleared this pathway before, so there's precedent. The not-so-good news: the pathway requires data showing the test is safe and effective, which means clinical trials and validation studies.
Vivoo's strategy of working with research partners makes sense in this context. They're gathering the data they'll need for regulatory approval. They're likely already in discussions with the FDA about what that approval pathway looks like.
Outside the US, the regulatory landscape varies. The EU has CE marking requirements. Other countries have their own medical device regulations. A truly global product needs to navigate all of these.
Regulation might sound like a burden, and in many ways it is. But it's also valuable. It means that when the product launches, you can have some confidence that it's been tested, that the claims are supported by evidence, and that there's oversight ensuring safety. Regulatory approval isn't a guarantee that a product is perfect, but it's a signal that independent bodies have reviewed the data and found it acceptable.
The regulatory status of the Flow Pad will be something to track as the product develops. Has it gotten FDA clearance? CE marking? What did that process reveal about effectiveness and accuracy? Those details matter for deciding whether to trust it.

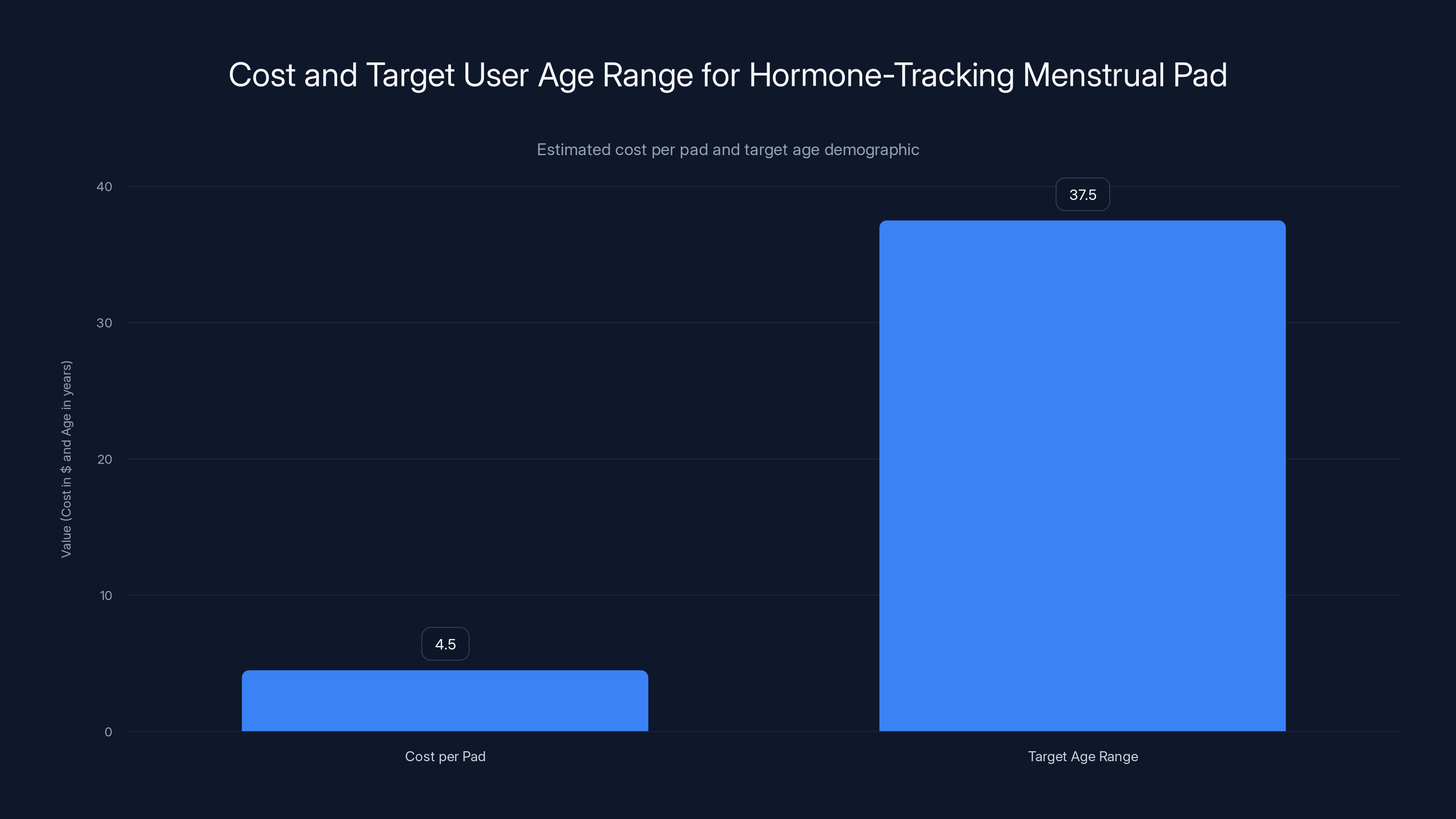
The hormone-tracking menstrual pad is priced between
How This Fits Into the Broader Wellness Tech Landscape
The Flow Pad doesn't exist in isolation. It's part of a much broader trend toward biomarker testing, home diagnostics, and personal health data collection.
Companies like Everlywell, Lets Get Checked, and others have made at-home testing convenient and normalized. You order a test online, collect a sample at home, mail it in, and get results through an app. That's become mainstream enough that insurance companies sometimes cover it.
Wearables that measure continuous biometrics—heart rate, sleep, activity, temperature—are becoming standard. Wearable manufacturers are starting to integrate more sophisticated health features. Apple's ECG function, for example, detects heart rhythm irregularities. That's a medical capability built into a consumer device.
The trend is clear: shifting diagnostic capability from clinics to home, from doctor-controlled to user-controlled, from episodic to continuous. The Flow Pad is another step in that direction.
But this trend comes with real tradeoffs. More data sounds great until you're managing it without professional interpretation. More testing sounds great until you get a result you don't understand and panic. More diagnostics sound great until you're diagnosing yourself with anxiety based on normal variation. The Flow Pad will likely include app-based guidance and interpretation, but that's only as good as the algorithm and the health literacy of the user.
The bigger picture: this is the emergence of a "wellness surveillance" landscape where your health is continuously monitored, quantified, and fed into platforms for analysis. That's powerful for people with genuine medical concerns who want better data about their health. It's potentially problematic for people seduced into excessive monitoring and self-diagnosis. The Flow Pad isn't uniquely problematic in this regard, but it's part of a larger pattern worth thinking critically about.
Real-World Use Cases: Who Benefits Most From the Flow Pad
Not everyone needs to know their FSH levels. Understanding who actually benefits from this product helps distinguish genuine utility from novelty.
The Person Trying to Conceive
Let's say you're thirty-eight, thinking about whether to try for a baby, and you want data about your fertility before committing to trying and potentially spending thousands on fertility treatment. You could use a Flow Pad to get a baseline FSH reading. If it's normal, you can move forward with trying. If it's elevated, it tells you your ovarian reserve isn't what it was at twenty-five, and that information might prompt you to start trying sooner rather than later, or to have a conversation with a fertility specialist about your options. That's genuinely useful information that's hard to get without professional intervention.
The Person With Irregular Cycles
If your periods are unpredictable—sometimes forty days apart, sometimes sixty—and you don't know why, FSH testing might help explain it. PCOS, thyroid issues, and approaching perimenopause all cause irregular cycles. FSH helps differentiate between them. Being able to test yourself rather than scheduling a doctor's appointment to get bloodwork is valuable.
The Person Managing PCOS
You've been diagnosed with PCOS. You're on treatment. You want to know if it's working. Traditional approach: three-month checkup with blood work. New approach: test yourself monthly or quarterly with a Flow Pad, see whether your FSH is trending in the expected direction, and only schedule a doctor's visit if something looks off. That's more empowering and cheaper.
The Person Approaching Menopause
You're forty-eight. Your periods have gotten irregular. You're having hot flashes. You don't know if it's early perimenopause or something else. FSH testing tells you. If it's rising, you can start planning for perimenopause with your doctor. You know what to expect. You can access treatment options. You're not in the dark anymore.
For each of these use cases, the Flow Pad provides genuine value by making hormone testing accessible and convenient. It's not that the test is better than traditional bloodwork—it's that it removes friction and cost, making testing actually happen rather than remaining on someone's todo list indefinitely.
But there are people for whom it's less useful. If you have regular periods and no symptoms, your FSH is almost certainly normal. Testing wouldn't tell you anything you don't already know. If you're already seeing a reproductive endocrinologist, they'll probably want you doing traditional blood work that includes multiple hormones and can include more sophisticated analysis. If you're pregnant or breastfeeding, FSH testing doesn't apply. The Flow Pad isn't a universal tool; it's a tool that solves specific problems for specific groups of people.
The Business Model: Sustainability and Accessibility Questions
At
The unit economics are interesting. Vivoo says they want to keep the pad under
Where the business scales is through recurring revenue and subscriptions. If someone uses a Flow Pad once a month for a year, that's twelve pads at
But user retention is the real question. Will people keep buying the Flow Pad month after month, or will they use it a few times and stop? People trying to conceive might use it for three to six months, then either get pregnant or move on. People managing PCOS might use it intermittently. The person with regular cycles and no concerns probably uses it once and realizes they don't need to repeat it.
That's why subscription and pack configurations make sense. Monthly subscriptions create predictable revenue and increase stickiness. Bundling the pads with app features or premium analysis might increase perceived value and willingness to pay. Partnerships with healthcare providers or insurance companies could drive bulk purchases and integrate the Flow Pad into actual healthcare workflows rather than positioning it purely as a wellness gadget.
The accessibility angle is real too. Vivoo is explicitly committed to keeping the price low and making this available to people regardless of economic status. That's admirable, but it's also a constraint on margins. To remain accessible while staying profitable, they need either scale, operational efficiency, or both.

The Bigger Picture: What This Means for Women's Health Tech
The Flow Pad represents a meaningful shift in how reproductive health technology might evolve. It's combining biomarker testing with an existing infrastructure—menstrual pads that people already use—in a way that's elegant and practical.
That approach could inspire other innovations. Could menstrual pads be used to detect other biomarkers besides FSH? Potentially. Could other bodily fluids—saliva, urine, sweat—be integrated into everyday products in similar ways? Almost certainly. We might see smart textiles that detect health biomarkers, diagnostic elements embedded in everyday personal care products, and a general shift toward ambient health monitoring that happens as a natural part of daily life rather than through active testing.
But there's also a cautionary tale here about the medicalization of normal biological processes. Yes, there are legitimate medical reasons to measure FSH. But there's also an incentive within wellness culture to create pathology where none exists, to encourage excessive testing, to make people worry about biomarkers that are likely fine. The Flow Pad could become a tool for health empowerment or a tool for health anxiety, depending on how it's used and promoted.
For that reason, the research validation and regulatory oversight matter. Having independent verification that the Flow Pad is accurate, that its results align with traditional testing, and that clinical studies show its utility for the intended uses is crucial. Marketing it as a tool for specific, legitimate health questions (fertility assessment, PCOS monitoring, perimenopause tracking) is appropriate. Marketing it as something everyone should use to optimize their reproductive health is not.
The healthcare system might also benefit from this innovation. Right now, FSH testing happens mostly in specialty settings (fertility clinics, OB-GYN offices) because it requires infrastructure and money to order and process tests. If FSH testing becomes something people can do at home, the data flows become different. Primary care physicians might have better baseline data when someone comes in with menstrual or hormonal concerns. The barrier to entry for researching reproductive health patterns drops significantly.
That could be genuinely valuable if the system is prepared to handle it. Physicians would need to know how to interpret home-generated FSH data. Apps would need to be aligned with clinical best practices rather than optimized for engagement above accuracy. Insurance systems would need to decide whether at-home FSH testing is covered, reimbursed, and integrated into medical workflows.
Historically, new healthcare technologies take longer to integrate into actual clinical practice than you'd expect. The Flow Pad might be in people's hands years before it's genuinely integrated into healthcare workflows. That doesn't make it useless, but it does mean the ultimate value might take time to realize.
The Limitations You Should Understand Before Using It
The Flow Pad is genuinely innovative, but it has real limitations worth understanding:
Single hormone, single timepoint: FSH is one hormone. Your reproductive system involves FSH, LH (luteinizing hormone), estrogen, progesterone, and others. A single FSH reading is useful but incomplete for comprehensive fertility assessment or hormonal diagnosis.
Accuracy validation pending: The product is still in early access. Peer-reviewed research comparing Flow Pad results to traditional FSH testing hasn't been published yet. You're early-adopting based on principle and promise rather than peer-reviewed evidence of accuracy.
App-based interpretation: Your results will be analyzed by an algorithm, not a physician. That interpretation is only as good as the algorithm and the data it was trained on. What if the app tells you something that contradicts what your doctor thinks?
No medical advice: The app can tell you your FSH level and provide general context, but it can't replace a medical consultation. If you have symptoms or are making medical decisions based on results, you still need to talk to a doctor.
Context dependency: FSH varies throughout your cycle, varies between cycles, and can vary based on stress, illness, exercise, and other factors. A single reading provides data, but it requires context to be truly meaningful.
Privacy tradeoffs: You're generating sensitive health data and sharing it with a company. That's a tradeoff with real privacy implications worth thinking through.
Supply and sustainability: The product is new. Will Vivoo continue producing it? Will they maintain the app? Will the company survive long-term? You're betting on that.
These limitations don't make the Flow Pad useless. They just mean you should approach it with realistic expectations. It's a tool that provides a specific type of useful information. It's not a comprehensive reproductive health solution.
Looking Forward: What's Next for Period Tech Innovation
If the Flow Pad succeeds, what does that mean for the future of period tech and reproductive health innovation?
We'll likely see more products that combine hygiene products with diagnostics. Tampons with biosensors. Panties with embedded microfluidic systems. Pads that measure multiple biomarkers, not just FSH. The technical barrier is falling, and once a concept works, iteration is rapid.
We'll probably see integration with broader health platforms. Vivoo data flowing into Apple Health or Google Health. Integration with fertility tracking apps that pull in FSH data automatically. Your reproductive health data becoming part of your broader health profile.
We might see clinical integration happen faster than traditional diagnostics. If the data is good and the value is proven, healthcare systems might push to make at-home FSH testing standard for certain populations. Women over thirty-five trying to conceive might automatically get the Flow Pad as part of preconception counseling. Women presenting with irregular periods might be offered Flow Pad testing before more invasive workup.
There will absolutely be pushback too. Privacy advocates will raise legitimate concerns. Bioethicists will ask uncomfortable questions about surveillance and medicalization. Insurance companies will debate whether at-home testing should be covered. Some physicians will resist information not generated in their labs. That's all normal and healthy.
The technology itself is only part of the story. How it's regulated, marketed, integrated, and culturally adopted will determine whether it becomes a genuinely useful health tool or a well-intentioned wellness gadget.
FAQ
What exactly is the Vivoo Flow Pad?
The Vivoo Flow Pad is a menstrual pad with embedded microfluidic channels and chemical reagents that measure follicle-stimulating hormone (FSH) levels in menstrual blood. You use it like a normal pad, and when you're ready to change it, you can read your FSH level directly from a windowed area on the back of the pad, or photograph it and use the Vivoo app for more detailed analysis and insights.
How does the Flow Pad measure FSH?
The pad contains two functional layers. The top layer has a capillary capture system that draws in menstrual blood and filters out particulates. The layer below contains stabilized chemical reagents that are designed to specifically interact with FSH molecules. When your blood meets these reagents, a chemical reaction occurs that produces a visible result. The intensity of the result corresponds to your FSH level, similar to how a pregnancy test shows intensity based on hormone concentration.
When should I wear the Flow Pad during my cycle?
Vivoo recommends wearing the Flow Pad on the second or third day of your menstrual cycle. This timing is important because FSH levels are most stable and standardized at this point in your cycle, which makes the reading more meaningful and comparable over time if you test in future cycles.
What does my FSH level actually tell me?
Your FSH level provides information about your ovarian reserve, hormonal status, and potential reproductive health concerns. Elevated FSH can indicate diminished ovarian reserve (fewer eggs remaining), conditions like PCOS, or the approach of perimenopause. For someone trying to conceive, elevated FSH might prompt fertility evaluation. For someone with irregular periods, it helps identify the underlying cause. For someone in their 40s, rising FSH suggests perimenopause is approaching. However, a single FSH reading is one data point, not a diagnosis.
Is the Flow Pad accurate compared to traditional blood tests?
Vivoo is currently working with research partners to validate the Flow Pad against traditional laboratory FSH testing. Peer-reviewed studies haven't been published yet, so independent confirmation of accuracy isn't available. Before relying on the product for medical decisions, look for published research comparing Flow Pad results to standard blood-based FSH tests from certified laboratories.
Who benefits most from using the Flow Pad?
The ideal candidates are people aged thirty to forty-five who are trying to conceive and want to assess their ovarian reserve, people with irregular periods looking to understand the hormonal cause, people managing PCOS who want to monitor hormone levels, or people suspecting they might be entering perimenopause. People with regular cycles and no reproductive health concerns, those already under the care of fertility specialists using traditional testing, and those not interested in hormone tracking probably won't find it useful.
How much does the Flow Pad cost?
Vivoo estimates the Flow Pad will cost between
When will the Flow Pad actually be available for consumers to buy?
As of early 2025, the Flow Pad is in early access with researchers, medical partners, and existing Vivoo users. Broader consumer availability hasn't been announced yet. The rollout is intentionally staged to allow for research validation, clinical integration, and regulatory compliance before mass market release.
What are the privacy concerns with using the Flow Pad?
The Flow Pad generates sensitive reproductive health data that will be transmitted to Vivoo's servers for analysis. Relevant privacy concerns include: who has access to your data, how it's stored and protected, how long it's retained, whether it could be sold or shared, how it might be used in the future, and whether it could affect insurance, employment, or other aspects of your life. Reproductive health data is particularly sensitive. Before using the product, you should review Vivoo's privacy policy and data security practices, which they should publish transparently before launch.
Can the Flow Pad diagnose medical conditions like PCOS or perimenopause?
No. The Flow Pad measures FSH levels and can contribute information that helps in diagnosis of these conditions, but a single hormone measurement can't diagnose anything. Conditions like PCOS require multiple hormone measurements, imaging, clinical evaluation, and medical interpretation. Perimenopause involves FSH measurement but also symptom assessment and medical judgment. The Flow Pad is a data-generating tool that supports medical decision-making, not a diagnostic tool itself.
How often should I use the Flow Pad?
Vivoo envisions the Flow Pad as a flexible tool. People trying to conceive might use it for a few months. People managing PCOS might use it monthly or quarterly to track progress. Someone checking their perimenopause status might use it intermittently over years. You don't need to use it every single period—the product is designed around the idea that people will use it when it's relevant to their current health priorities and questions.
The Bottom Line: Innovation With Important Caveats
The Vivoo Flow Pad is a genuinely clever innovation that removes meaningful barriers to hormone testing. It combines an existing hygiene product that billions of people use with diagnostic capability that typically requires doctor visits and lab infrastructure. That combination is useful and has real potential to improve access to reproductive health information.
But it's also early-stage technology that requires research validation, careful regulatory navigation, and thoughtful integration into actual healthcare practice to reach its potential. The fact that it exists is notable. The fact that it works in theory is encouraging. But the fact that peer-reviewed research and regulatory approval processes are still pending means the full story hasn't been written yet.
For people asking specific questions—Am I about to enter perimenopause? Should I be worried about my fertility? Is my PCOS under control?—the Flow Pad could provide valuable information right now, assuming it proves accurate in validation studies. For everyone else, it's a tool worth monitoring but probably not essential.
The bigger picture is the trend it represents. Home diagnostics, biomarker testing, reproductive health transparency, and personal health data collection are all accelerating. The Flow Pad is part of that acceleration. How it's integrated, validated, regulated, and used will determine whether it's a meaningful step forward for reproductive health or a clever wellness gadget that looks good on the surface but doesn't drive real change.
Give it time. Let the research validation happen. Let regulatory processes play out. Let early users provide real-world feedback. By 2026 or 2027, we'll know whether the Flow Pad is actually as useful as it promises, or whether it's innovation that works in the lab but doesn't translate to the real world. Until then, it's worth watching with interest and appropriate skepticism.

Key Takeaways
- The Vivoo FlowPad integrates FSH hormone measurement directly into menstrual pads, eliminating the need for doctor's office visits for fertility hormone testing
- At 5 per pad, the FlowPad costs 95% less than traditional FSH blood tests that typically cost300
- The pad is designed for people aged 30-45 tracking fertility, managing PCOS, or monitoring for perimenopause transitions
- FSH measurement requires careful interpretation because hormone levels fluctuate throughout the cycle—the FlowPad's usefulness depends on consistent timing and app-based guidance
- As biotech innovation continues embedding diagnostics into everyday products, regulatory validation and privacy protection become increasingly important for consumer health devices
Related Articles
- At-Home Hormone Testing: How the Mira Ultra4 Changes Fertility & Wellness [2025]
- Withings Body Scan 2: The Ultimate Smart Scale for Longevity Tracking [2026]
- Best Meditation Apps [2025]: Science-Backed Mindfulness Guide
- 33 Top Health & Wellness Startups from Disrupt Battlefield [2025]
- Best Journaling Apps in 2025: Complete Guide to Digital Reflection [2025]
![Vivoo FlowPad: Smart Menstrual Pad Hormone Testing [2025]](https://tryrunable.com/blog/vivoo-flowpad-smart-menstrual-pad-hormone-testing-2025/image-1-1767670627208.jpg)



