Noninvasive Blood Glucose Monitors: The Wearable Breakthrough [2025]
For decades, diabetes management has meant needles. Constant, painful needle sticks multiple times a day. Fingers pricked until they're scarred. Kids crying during routine monitoring. Old people struggling with trembling hands trying to get a clean drop of blood.
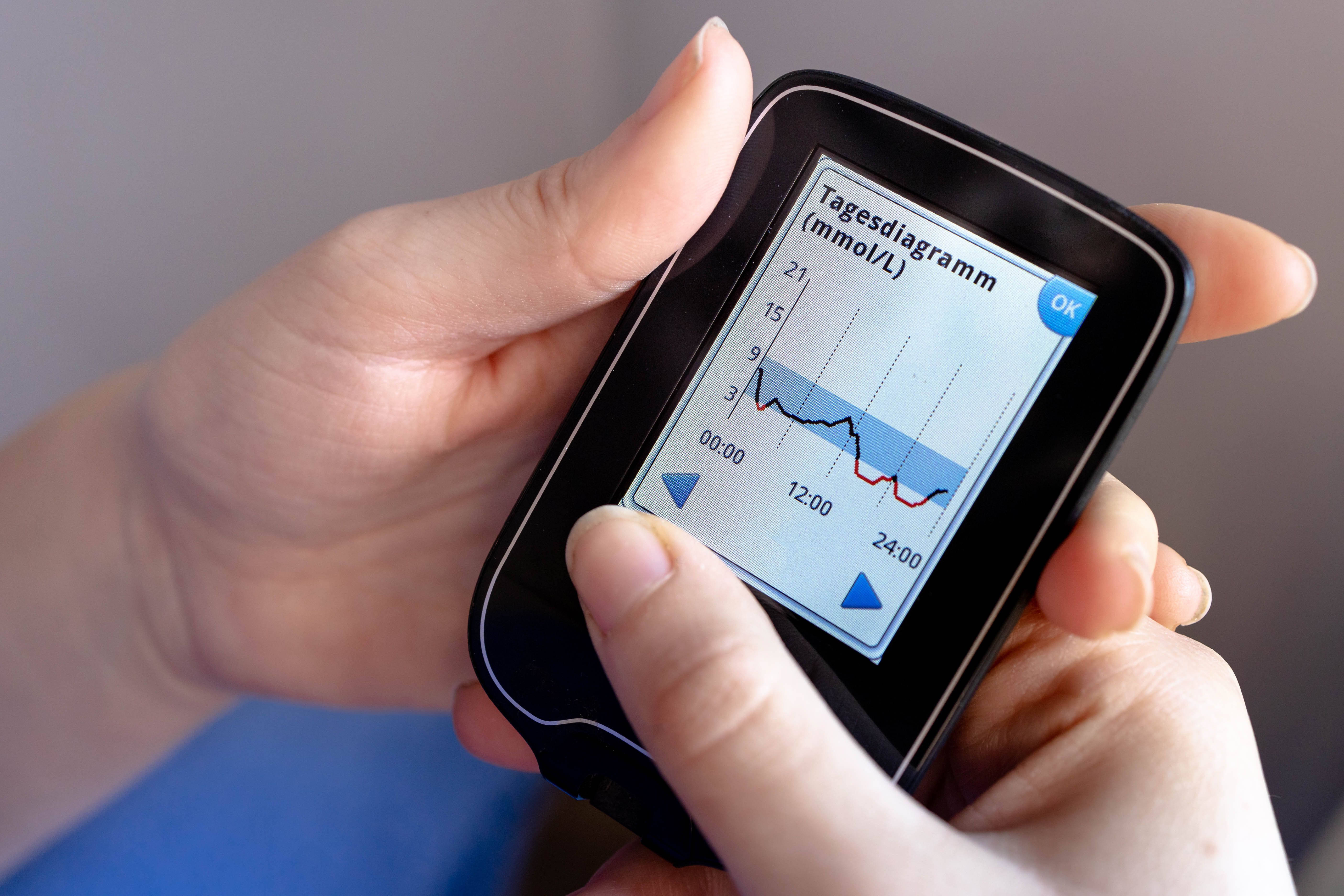
Then continuous glucose monitors (CGMs) came along and made things better. But they still required inserting a sensor under your skin. Still felt invasive. Still left people with visible devices that broadcast their medical condition to anyone nearby.
Now, after years of false starts and broken promises, noninvasive glucose monitoring is actually happening. Not in the next decade. Not in a prototype that might work someday. Right now. At CES 2025, major companies are finally showing working devices that measure blood sugar without ever breaking your skin, as highlighted by Sensura's showcase.
The shift matters more than you might think. It's not just about comfort, though that's huge. It's about making diabetes management accessible to people who couldn't handle it before. It's about eliminating a barrier that kept millions from getting the continuous monitoring their bodies desperately needed. And it's about proving that sometimes, when an industry says something's impossible, they just haven't been looking hard enough.
Here's what's actually happening in glucose monitoring right now, why it took so long to get here, and what comes next.
TL; DR
- Noninvasive glucose monitoring is real: Pre Evnt Isaac and similar devices measure blood sugar through breath analysis instead of blood draws or skin insertion.
- It works by detecting biomarkers: Devices measure volatile organic compounds like acetone in your breath that correlate with blood glucose levels, as discussed in a recent study.
- FDA approval is coming: Clinical trials are underway at major research institutions with regulatory pathways actively being pursued, as noted in Cureus.
- Game-changing for kids and elderly: The technology eliminates the needle pain that made consistent monitoring nearly impossible for young children and people with needle anxiety.
- Not yet in smartwatches: While Apple and other companies tried integrating glucose monitoring into wearables, these new devices work as standalone necklace-worn sensors instead.

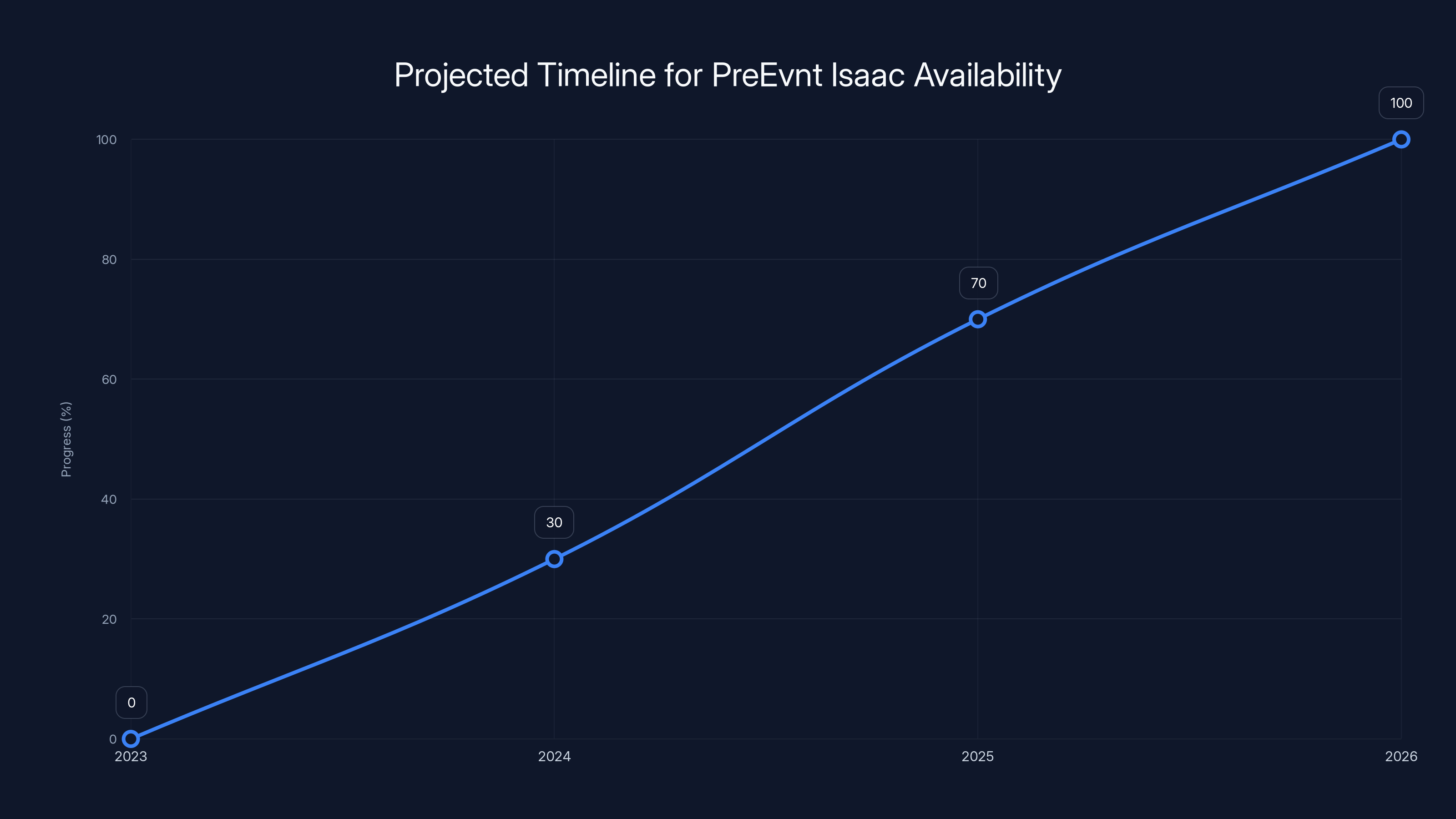
The PreEvnt Isaac is expected to complete clinical trials and FDA submission by 2025, with potential market availability in early 2026. Estimated data based on current projections.
Why Glucose Monitoring Has Been So Hard
Measuring blood glucose seems straightforward: test the blood, read the number, adjust treatment. In reality, it's one of the most technically challenging measurements in all of medicine.
Your blood chemistry changes constantly. Glucose levels spike and crash within minutes depending on food, stress, sleep, exercise, medications, and a hundred other variables. For diabetics, this constant fluctuation is the core problem they're trying to manage. They need to know not just their glucose level right now, but the direction it's heading.
Traditional finger-stick testing captures only a single moment in time. You prick your finger, get a number, and that's it. By the time you've finished breakfast, your glucose has changed dramatically. This is why doctors pushed for continuous monitoring. But getting a sensor under your skin every time you need monitoring is still invasive, still painful, and creates barriers that keep many people from doing it as often as they should.
Why couldn't smartwatch makers just use optical sensors to measure glucose through the skin? Apple, Google, Samsung, and dozens of smaller companies tried. The physics work in theory. Glucose absorbs light at specific wavelengths. If you can measure how that light is absorbed through the skin, you should be able to calculate glucose levels.
Except skin is incredibly complex. Different people have different skin pigmentation, thickness, blood flow, and composition. What works for one person gives garbage results for another. Add in sweat, temperature changes, movement, and other variables, and optical glucose measurement through skin becomes nearly impossible to make work reliably. Apple and other companies spent years and millions of dollars chasing this approach before essentially giving up.
Companies explored other approaches. Electromagnetic sensors. Thermal imaging. Ultrasound. None of them achieved the accuracy and reliability needed for medical use. The FDA requires glucose monitors to be extremely accurate because errors can lead to dangerous treatment decisions. Too high a reading and diabetics might not take enough insulin. Too low and they might overdose.
Then Pre Evnt discovered something different. Instead of trying to measure glucose directly through the skin, what if you could measure something that correlates with glucose?
The Breath Biomarker Discovery
Bud Wilcox, Pre Evnt's founder, had a deeply personal motivation. His grandson was diagnosed with type 1 diabetes at age two. Imagine trying to get a two-year-old to sit still for blood glucose testing multiple times a day. Imagine the tears, the fear, the trauma of constant needle sticks.
Wilcox realized that diabetics often develop a distinctive sweet, fruity smell on their breath. This "acetone breath" is a well-documented symptom that occurs when the body breaks down fat for energy instead of glucose, creating ketone bodies like acetone. The symptom has been known for centuries. Medieval physicians could diagnose diabetes partly by smelling patients' breath.
But Wilcox saw this differently. What if acetone and other volatile organic compounds (VOCs) in breath could serve as reliable biomarkers for glucose levels? You couldn't measure glucose directly through breath, but you could measure these compounds and use that data to infer glucose levels.
The approach had advantages. Everyone breathes constantly. Breath is easily accessible. You don't need to break the skin. And the chemistry is well-established. Volatile organic compounds are real, measurable, and exist in specific concentrations based on metabolic state.
The challenge was creating sensors sensitive enough to detect these compounds accurately, and then building AI models that could correlate VOC patterns with actual glucose levels. It wasn't just hardware. It was biochemistry meets machine learning meets clinical validation.
Pre Evnt spent years developing the technology. They created a small sensor that could reliably detect and measure specific volatile organic compounds. They built algorithms trained on thousands of hours of glucose monitoring data to correlate VOC patterns with actual blood glucose levels. They ran laboratory tests, animal studies, and finally moved to clinical trials with real patients.
The first public demo at CES 2025 shocked the industry. It actually worked.
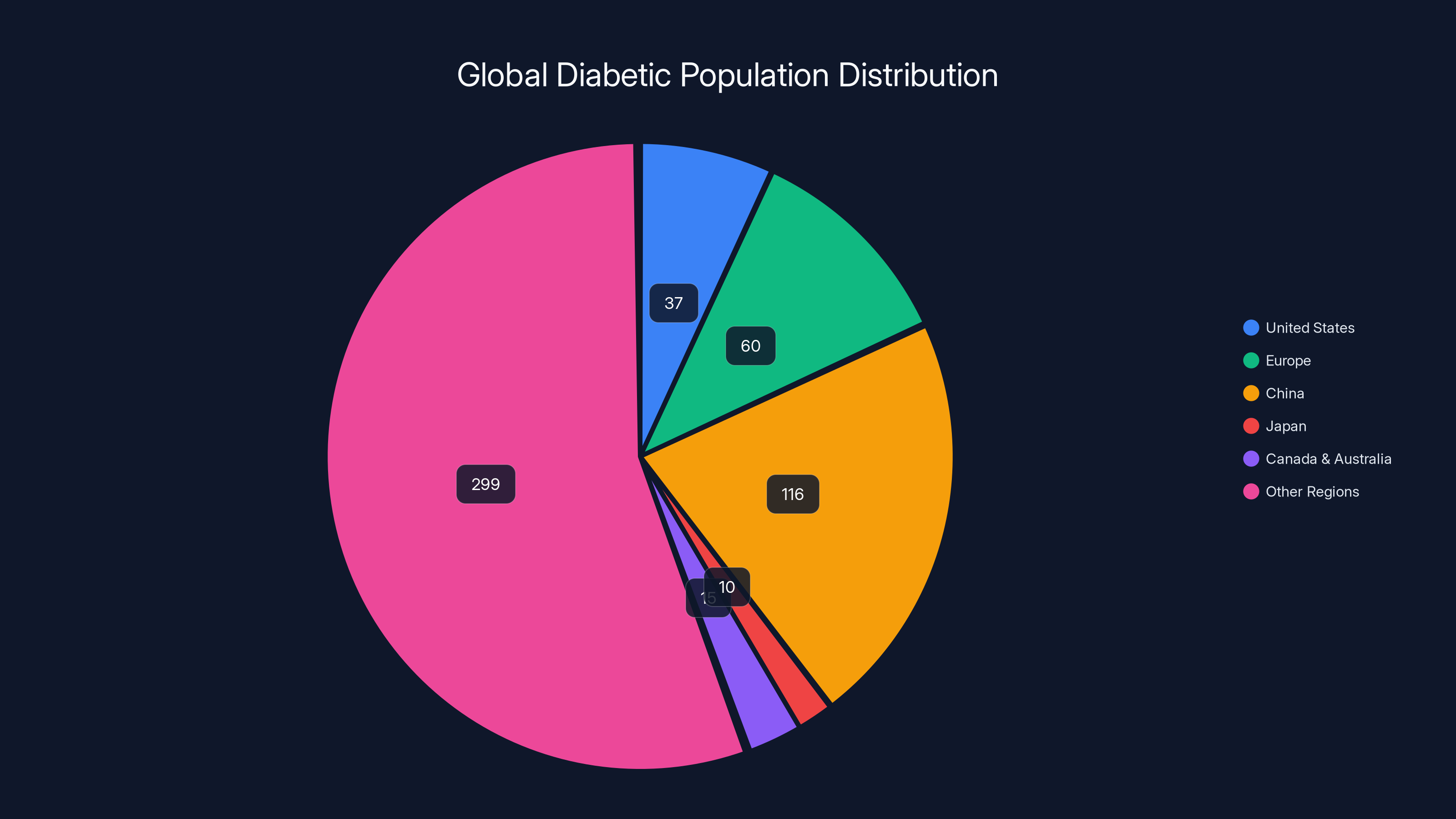
Estimated data shows that China has the largest diabetic population, followed by other regions, highlighting the global market potential for noninvasive monitoring devices.
How Pre Evnt Isaac Actually Works
The Isaac is small. About the size of a quarter, maybe slightly bigger. It's not some bulky device you have to carry around. You wear it on a necklace or keep it in a small pouch. The design matters because the target market includes young children who can't be expected to manage a large device.
Inside the Isaac is a sophisticated sensor array. When you breathe onto it, the device captures the volatile organic compounds in your breath. The sensor measures the concentration of multiple compounds, not just acetone. It's building a chemical fingerprint of your metabolic state.
That data gets processed locally on the device using trained machine learning models. The algorithms were built by feeding the system thousands of hours of real glucose monitoring data from people with type 1 and type 2 diabetes. The algorithms learned the patterns. They figured out which VOC combinations correlate with which glucose levels.
Within seconds, the Isaac calculates your current blood glucose level. It transmits that to a companion smartphone app via Bluetooth. The app logs the measurement, tracks your glucose over time, and can alert you if levels are dangerously high or low.
There's even a social component. The app lets you share alerts with emergency contacts. If your glucose drops dangerously low, the device can automatically notify designated people that you need help. For people with diabetes, especially young children and elderly people who might become confused or incapacitated during hypoglycemic episodes, this is genuinely life-saving.
The frequency matters. Unlike traditional glucose meters that give you one number per test, Isaac provides continuous tracking. The idea is that you'd check multiple times throughout the day, similar to how CGM users get constant readings. The more frequent measurements, the more complete picture of your glucose patterns.
One concern people immediately ask: how do you know the measurements are accurate? Especially during clinical trials, Pre Evnt had to prove that the Isaac's readings matched traditional laboratory glucose measurements. The trials compare Isaac readings directly against gold-standard glucose testing methods.
The science here is sound. Volatile organic compounds in breath genuinely do correlate with metabolic state and glucose levels. Research going back decades shows this relationship. What Pre Evnt did was engineer sensors sensitive enough to detect the differences, and build algorithms precise enough to make medical-grade predictions from those measurements, as supported by Nature's findings.
Clinical Trials and FDA Pathway
Pre Evnt didn't just build a device and hope it would work. They've been systematically running clinical trials to validate the technology. The trials started with adolescents diagnosed with type 1 diabetes—some of the most challenging patients because type 1 diabetes is more volatile and requires tighter glucose control.
The trials are being conducted at major research institutions including Indiana University. Researchers are comparing Isaac readings with continuous monitoring data from standard CGMs and laboratory glucose measurements. They're testing different demographics, different time of day, different meal types, different stress levels—all the variables that affect glucose.
What's particularly important is that Pre Evnt is working directly with the FDA through what's called a "de novo application." This pathway is used for novel devices that don't fit into existing categories. Fredrick Brooks, Pre Evnt's director of health technologies, explained that the FDA has been "very interested and understanding" of the approach.
That matters. The FDA is basically saying: we've never seen glucose monitoring done this way before. We're going to work with you to figure out what validation standards apply. What evidence do we need? What accuracy thresholds are necessary? How do we ensure this is safe and effective?
The FDA's receptiveness is partly because the need is so obvious. Millions of diabetics worldwide aren't getting adequate glucose monitoring because traditional methods are too invasive or inconvenient. If Pre Evnt can deliver a safe, accurate, noninvasive solution, regulators understand the public health benefit.
The timeline is realistic. Fredrick Brooks indicated that FDA regulatory review is expected in the upcoming year. Once submitted, review typically takes 2-6 months for devices like this, though complex novel devices sometimes take longer. If all goes well, Pre Evnt could have regulatory approval by late 2025 or early 2026.
What happens after approval? Typically, the device would launch first to healthcare providers. Doctors would prescribe it for patients, insurance might start covering it, and gradually it would become available through direct consumer purchase. Or Pre Evnt might take a different route and sell direct to consumers immediately. Different companies handle post-approval launch differently.
Why This Changes Everything
You might be thinking: okay, it's a new way to measure glucose. Why does it matter? People already have continuous glucose monitors.
But here's the thing. Current CGMs still require a sensor insertion under the skin. Yes, it's smaller than finger sticks. Yes, you only have to insert a new sensor every 10-14 days instead of pricking your finger 4-8 times daily. But it still hurts. It still leaves a mark. It's still visibly noninvasive in the literal sense, but it's invasive in the everyday sense of your life.
For a two-year-old, the difference between finger sticks and subskin sensors is huge, but it's still traumatic. For someone with severe needle anxiety, a sensor insertion is still terrifying. For elderly people with trembling hands or arthritis, even sensor insertions are difficult.
Noninvasive breath-based monitoring eliminates all of that. No pain. No blood. No insertion. No visible device on your body broadcasting that you have diabetes. You just breathe into a device a few times a day. That's it.
The quality of life improvement is staggering. Imagine being a parent of a diabetic child. Right now, you're doing finger sticks or managing sensor insertions multiple times daily. That's stressful. That's emotionally difficult. Now imagine that your child can just breathe into a device and get their glucose level. The resistance to monitoring disappears. The trauma disappears.
For adults, the story is similar. People with diabetes often skip glucose monitoring because it's inconvenient or painful. This behavior leads to worse health outcomes. With a noninvasive option, more people will monitor more consistently. Better adherence means better control. Better control means fewer complications.
There's also an accessibility angle. In countries where healthcare is limited, where people don't have easy access to insulin or ongoing diabetes management, a device that requires no disposable lancets or sensors could be transformative. You need the device itself, but you don't need boxes of consumable supplies. That changes the economics of diabetes management globally.
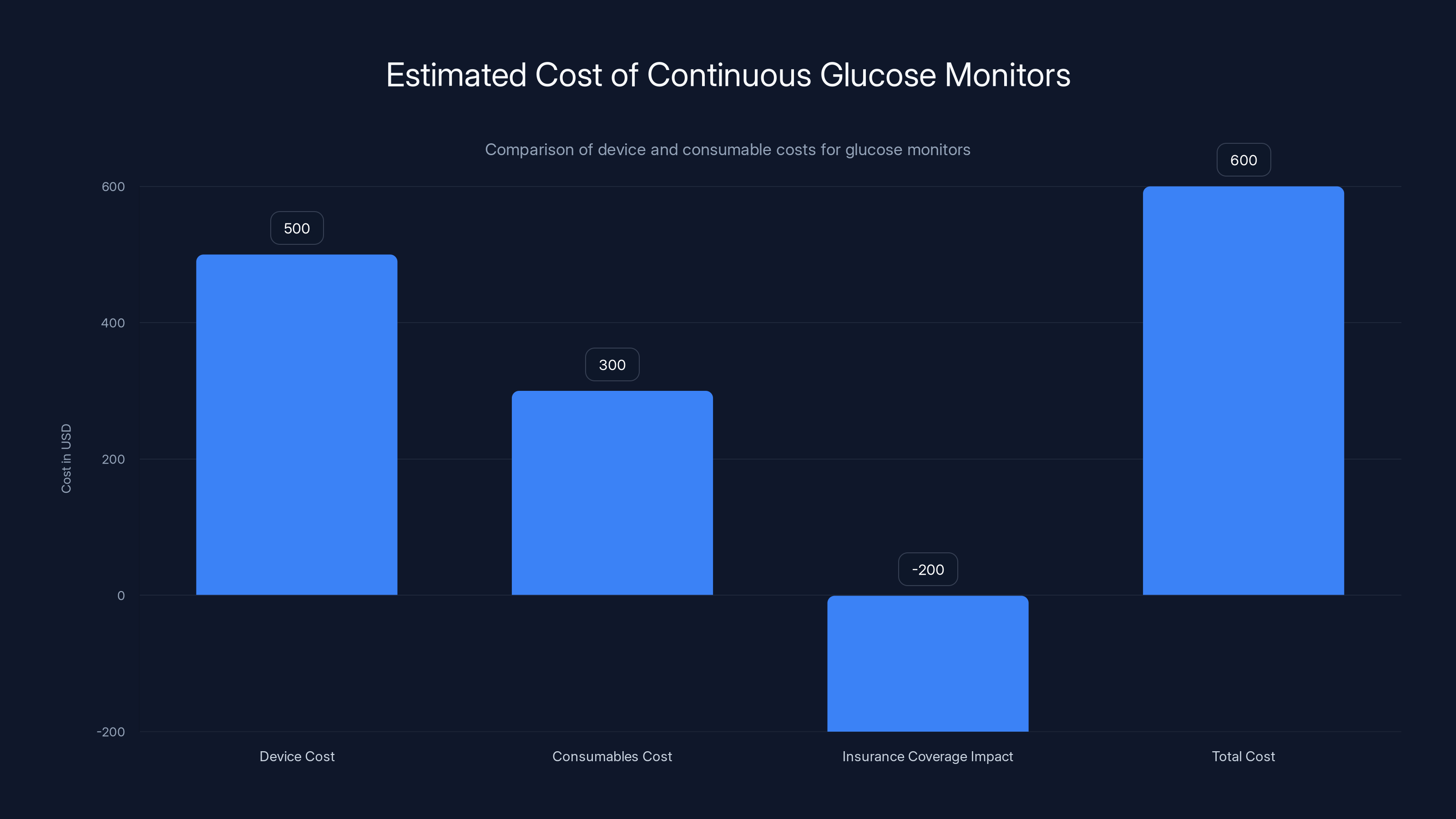
Estimated data shows that while device costs might be around
The Competition Heating Up
Pre Evnt isn't the only company pursuing noninvasive glucose monitoring. Once Pre Evnt demonstrated that breath-based measurement was actually viable, other companies started exploring similar approaches. Some are using different biomarkers. Some are using different sensor technologies. But the race is on.
Microsoft has been exploring glucose measurement technologies and filed patents related to optical glucose monitoring. Google X, now called X Development, has experimented with various approaches including contact lens glucose sensors. Neither has brought a product to market yet, but both have resources and expertise.
Smaller startups are also entering the space. Some are pursuing approaches similar to Pre Evnt. Others are trying different paths entirely. The competitive landscape will accelerate innovation, which typically drives down costs and improves accuracy.
What's interesting is that the established CGM companies haven't been as aggressive in pursuing truly noninvasive options. Companies like Abbott (which owns Free Style), Dexcom, and Medtronic have huge invested interest in the current sensor-based model. Switching to a completely different technology might cannibalize their existing business. But if a competitor brings a truly superior noninvasive solution to market, their customers will switch.
This is the classic innovator's dilemma. The incumbents control the market but are invested in the old technology. The insurgents are hungry to disrupt. Eventually, the market favors the better solution.
Integration with Health Data Ecosystems
One thing that makes modern glucose monitoring attractive is integration with other health data. Your smartwatch knows your activity level. Your phone knows your location and stress levels. Your calendar knows when you're at the gym or eating lunch. Glucose monitoring doesn't exist in isolation.
Pre Evnt's Isaac app is being designed to integrate with other health tracking data. The current version in development lets users log meals on a timeline. But the vision is bigger. Imagine the app correlating your glucose response with specific foods, your activity level, your sleep, your stress. Machine learning could start identifying personal patterns.
This is where the technology gets genuinely powerful. Not just for diagnosis, but for personalized optimization. You could see that your glucose is more stable when you eat protein before carbs. You could see that your response to a specific food changes depending on how much you slept. You could get specific, personalized recommendations based on your unique physiology.
Integration with platforms like Apple Health, Google Fit, and Fitbit's data ecosystem would extend the value even further. Imagine your smartwatch seeing your glucose level drop and automatically suggesting you take a break or recommending a snack. Imagine your smart home responding to your glucose status by adjusting temperature or lighting.
These might sound like sci-fi features. But they're all technically feasible once you have reliable, continuous glucose data. Companies like Runable are already pioneering AI automation platforms that could help synthesize health data and generate personalized wellness reports, dashboards, and recommendations. As these technologies mature, the ecosystem around diabetes management will become increasingly sophisticated.
Why Apple Watch Glucose Monitoring Never Happened
For years, Apple has been working on glucose monitoring for the Apple Watch. The company filed patents, hired researchers, invested substantial resources. Yet the feature still hasn't appeared in any shipping product. Why?
The technical challenges are real, but the regulatory challenges might be bigger. Bringing a medical device to market in the US requires FDA approval. That approval is rigorous, expensive, and time-consuming. It's the kind of project that takes 5-10 years and costs tens of millions of dollars.
Apple could afford this. But Apple's culture is different from medical device companies. Apple launches products when they're polished and perfect. Medical device companies launch products when they work and are safe, even if they're not perfect. The worlds don't align well.
Also, integrating glucose monitoring into a smartwatch that's designed for fitness tracking creates complications. The watch already does optical heart rate monitoring. Adding optical glucose monitoring might create interference or accuracy issues. Apple would need to completely redesign the optical sensor stack.
Meanwhile, dedicated devices like Isaac face fewer complications. They're single-purpose devices designed specifically for glucose monitoring. They can optimize every aspect for accuracy and reliability. They don't have to compromise to fit into a device designed for fitness and notifications.
This is why we might see noninvasive glucose monitoring arrive in dedicated devices or specialized wearables before we see it in mainstream smartwatches. Sometimes the best solution isn't the most integrated one.

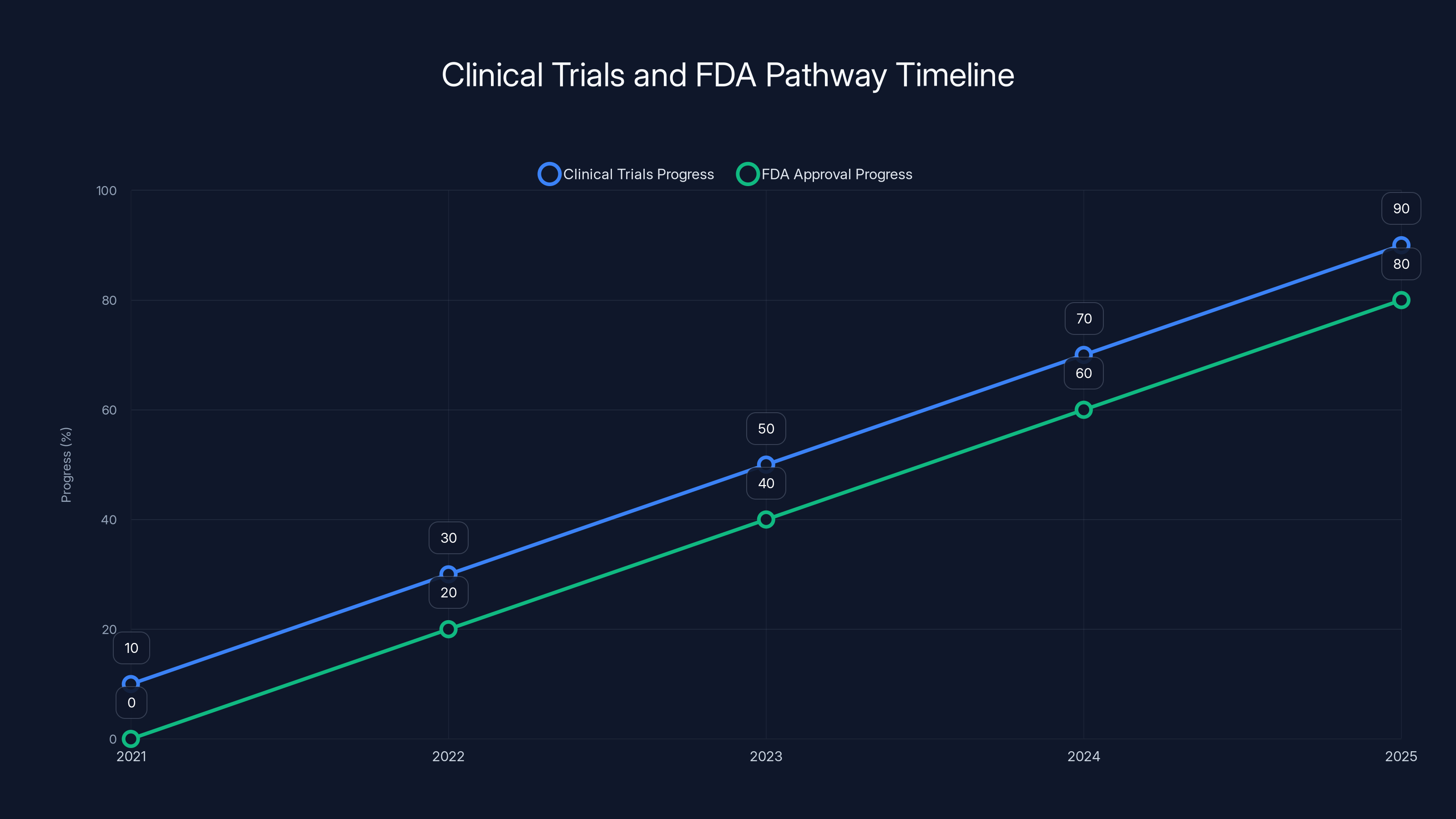
Estimated data shows the progress of clinical trials and FDA approval for PreEvnt's device from 2021 to 2025. The timeline suggests significant advancements in both areas, aiming for near completion by 2025.
The Metabolic Tracking Trend
Glucose monitoring isn't happening in a vacuum. There's an entire trend around metabolic tracking and optimization. GLP-1 agonists like Ozempic and Wegovy have exploded in popularity for weight loss. People want to understand their metabolism. They want to optimize their performance.
This created huge demand for glucose monitoring among non-diabetics. People without diabetes want to see how different foods affect their glucose. They want to understand their individual metabolic response. This drove demand for continuous glucose monitors, which were traditionally prescribed only for diabetics.
Fitness companies are capitalizing on this trend. Garmin added AI-powered nutrition tracking to its premium Connect+ subscription. Oura's ring lets you photograph meals and analyze them. Amazfit even created a prototype camera that records what you eat and how much time you spend eating.
Some of this is helpful. Understanding your personal glucose response to foods is genuinely useful information. It can help you make better food choices, manage energy levels, and lose weight if that's your goal.
But there's a darker side. The obsessive focus on optimizing every aspect of metabolism can create anxiety and orthorexia (obsessive concern about eating only "perfect" foods). Not everyone benefits from constant metabolic feedback. Some people become psychologically unhealthy when they focus too intensely on optimizing biometrics.
With that context, it's worth appreciating what Pre Evnt is doing. The Isaac wasn't created to optimize athletic performance or help wealthy people obsess over metrics. It was created because a young child was suffering. The technology serves a medical purpose. Yes, it might eventually be used for non-medical optimization. But its foundation is therapeutic, not hedonistic.
The Device Design Philosophy
One thing that struck observers at CES was how thoughtfully designed the Isaac is. It's small because it needs to work for children. It's wearable as a necklace because kids lose things constantly. The companion app is simple because parents need to understand it quickly.
This is the opposite of how some medical devices are designed. Too many medical devices look like they were engineered by people who've never actually used them. The interfaces are confusing. The hardware is clunky. The design assumes the user is a healthcare professional, not a person trying to manage their own health.
Pre Evnt clearly thought about real-world usage. A necklace is perfect for kids because you can't lose it as easily as a separate device. Small size is perfect because it's portable and doesn't feel burdensome. Breath-based interaction is perfect because it's non-threatening and requires no training.
This design thinking will become increasingly important as more noninvasive devices launch. The technology is only half the battle. The other half is creating devices that people actually want to use every day. Form factor, interface design, companion app experience—these all matter as much as raw accuracy.
If Pre Evnt can maintain this design focus through the path to FDA approval, they'll have a significant advantage over competitors who are purely focused on technical specs.
Regulatory Landscape and Global Approval
The FDA approval pathway in the US is just the beginning. Medical devices sold globally need approval in multiple jurisdictions. The EU has its own CE marking process. China has its own requirements. Japan, Canada, Australia, and other countries all have their own regulatory frameworks.
For a company like Pre Evnt, navigating this global regulatory landscape is complex but necessary. The potential market is huge. An estimated 537 million adults worldwide have diabetes. In the US alone, over 37 million people have diabetes. Even if only 10% of the diabetic population eventually switches to noninvasive monitoring, that's tens of millions of potential customers.
The regulatory process varies significantly by region. Some countries are more conservative and require extensive clinical data. Others are more flexible if a device has been approved elsewhere. Typically, a US FDA approval helps smooth the path in other developed markets.
In developing countries, the regulatory environment is different. Some countries have lenient device approval processes. Others have no functional regulation. This creates opportunities for companies to reach underserved markets but also raises ethical questions about whether devices should launch in countries without proper oversight.
Pre Evnt's approach seems methodical and thorough. They're doing clinical trials with diverse patient populations. They're working transparently with the FDA. They're building the scientific evidence necessary for global approval. This approach takes longer, but it builds credibility and ensures the device can be sold broadly.
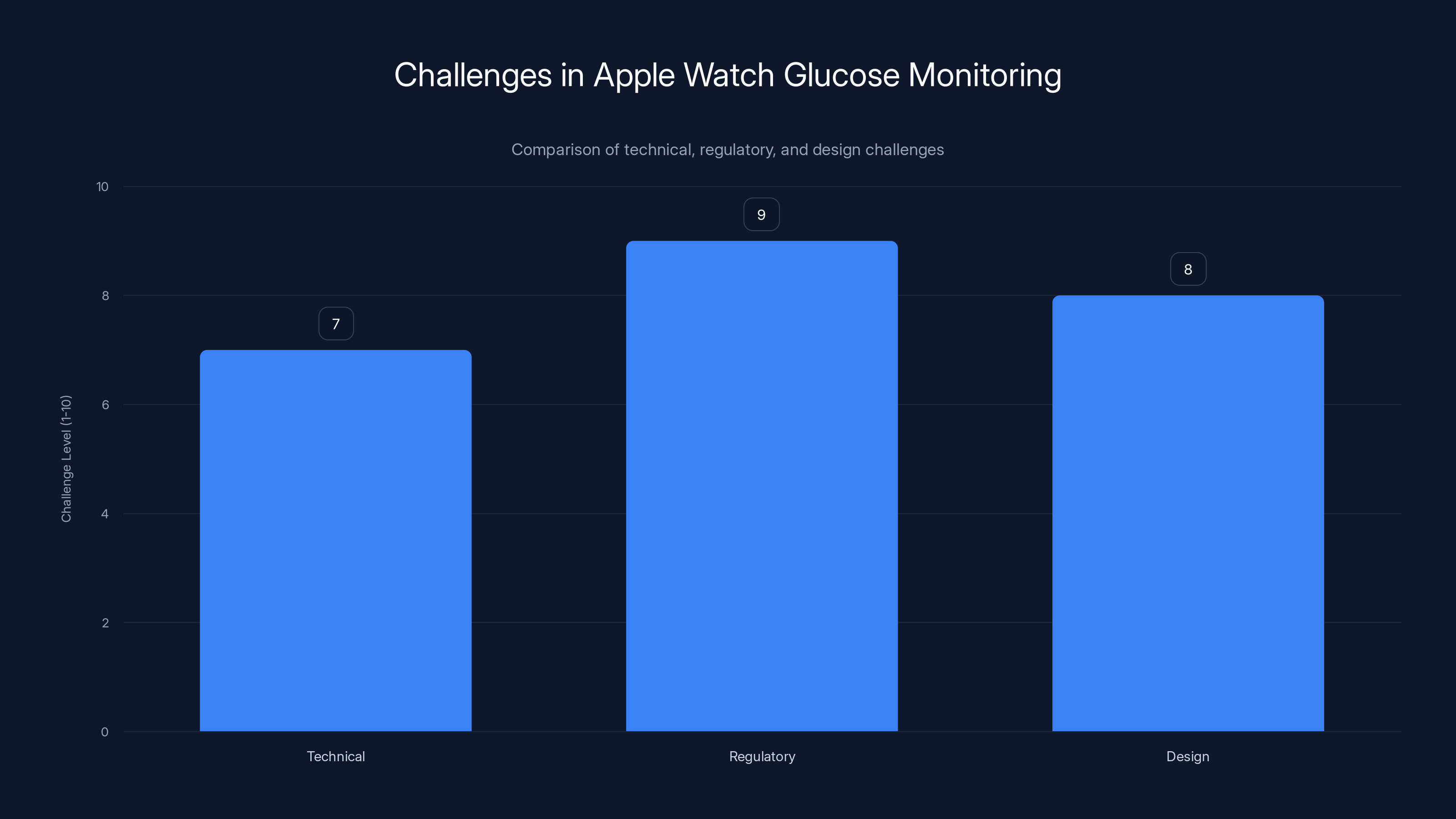
Regulatory challenges are the most significant barrier for Apple in implementing glucose monitoring in the Apple Watch, followed closely by design and technical challenges. Estimated data.
Privacy and Data Security Considerations
Continuous glucose monitoring generates sensitive health data. Your glucose levels, meal timing, activity patterns—this is intimate information that reveals a lot about your life. How is this data protected?
The Isaac stores glucose readings on your smartphone via a companion app. The app presumably syncs data to cloud servers so you can access it from different devices and Pre Evnt can analyze usage patterns. This creates privacy and security questions.
Who owns the data? Is Pre Evnt storing it securely? Could health insurers or employers access it? Could it be breached? These aren't hypothetical concerns. Medical device data breaches happen regularly. In 2023, Dexcom CGM users reported that hackers accessed their account data.
As noninvasive monitoring devices proliferate, privacy standards need to keep pace. HIPAA in the US provides some protection, but many direct-to-consumer devices operate in gray areas. Regulations like GDPR in Europe provide stronger privacy protections than many US state laws.
Pre Evnt hasn't published extensive information about their data security practices yet. As they move toward commercial launch, these policies will become increasingly important. Users need clear information about what happens to their data, who can access it, and how it's protected.
Companies that handle this well will gain competitive advantage. Users will choose devices from companies they trust with their health data. This creates incentive for Pre Evnt and competitors to implement best-in-class security and privacy practices.
Cost and Accessibility
One question everyone asks: how much will this cost?
Pre Evnt hasn't announced final pricing. But we can make educated guesses based on comparable devices. Current continuous glucose monitors cost patients anywhere from a few hundred to several thousand dollars depending on insurance coverage. The sensors themselves cost money, and the readers cost money.
The Isaac presumably requires the device itself (probably a few hundred dollars) and might require disposable sensor cartridges for the breath sampling element. If each breath sample requires a new cartridge (similar to how lancets require replacement), the long-term cost could add up.
Alternatively, Pre Evnt might design the device to be reusable without expensive consumables. This would be the ideal scenario for accessibility and sustainability. But it's unclear if the design supports this.
Cost is critical for accessibility. In the US, insurance might eventually cover noninvasive glucose monitoring for diabetics. But initial coverage is never guaranteed. For non-diabetics using it for optimization, coverage is unlikely. Cost will determine whether the technology remains a luxury item or becomes broadly accessible.
Globally, the cost question is even more important. In developing countries where diabetes rates are rising dramatically but healthcare access is limited, an expensive noninvasive monitor might be inaccessible to exactly the people who need it most.
Pre Evnt's business model will partially determine outcomes here. If they price the Isaac aggressively to drive adoption, they might capture market share but sacrificed margin. If they price for maximum profitability, they might limit addressable market. The right balance depends on manufacturing costs and competitive pressure.
Future of Glucose Monitoring Technology
Even as Pre Evnt moves toward FDA approval, the technology landscape continues evolving. What comes after breath-based VOC measurement?
Some researchers are exploring sweat-based glucose sensing. Your sweat contains glucose that correlates with blood glucose. Sensors that measure glucose in sweat could be embedded in smartwatch bands or clothing. The advantage is that sweat is continuously produced during activity, enabling passive monitoring.
Others are pursuing saliva-based measurement. Saliva also contains glucose and could potentially be measured with minimally invasive sensors.
There's also renewed interest in optical sensing. The approaches that failed for smartwatches might work better with dedicated sensor hardware that's optimized purely for glucose measurement rather than trying to fit optical sensing into a device designed for fitness tracking.
Non-invasive electromagnetic and spectroscopic approaches are being explored by various research groups. Some might eventually prove viable.
Machine learning and AI will accelerate this entire field. As more devices produce more glucose data, algorithms get better at correlating various biomarkers with actual glucose levels. Over time, the indirect approaches that currently seem uncertain might become as accurate as direct measurement.
The trajectory is clear. Noninvasive glucose monitoring is transitioning from "impossible" to "possible" to "practical" to "ubiquitous." It will take years to get there, but the direction is settled.
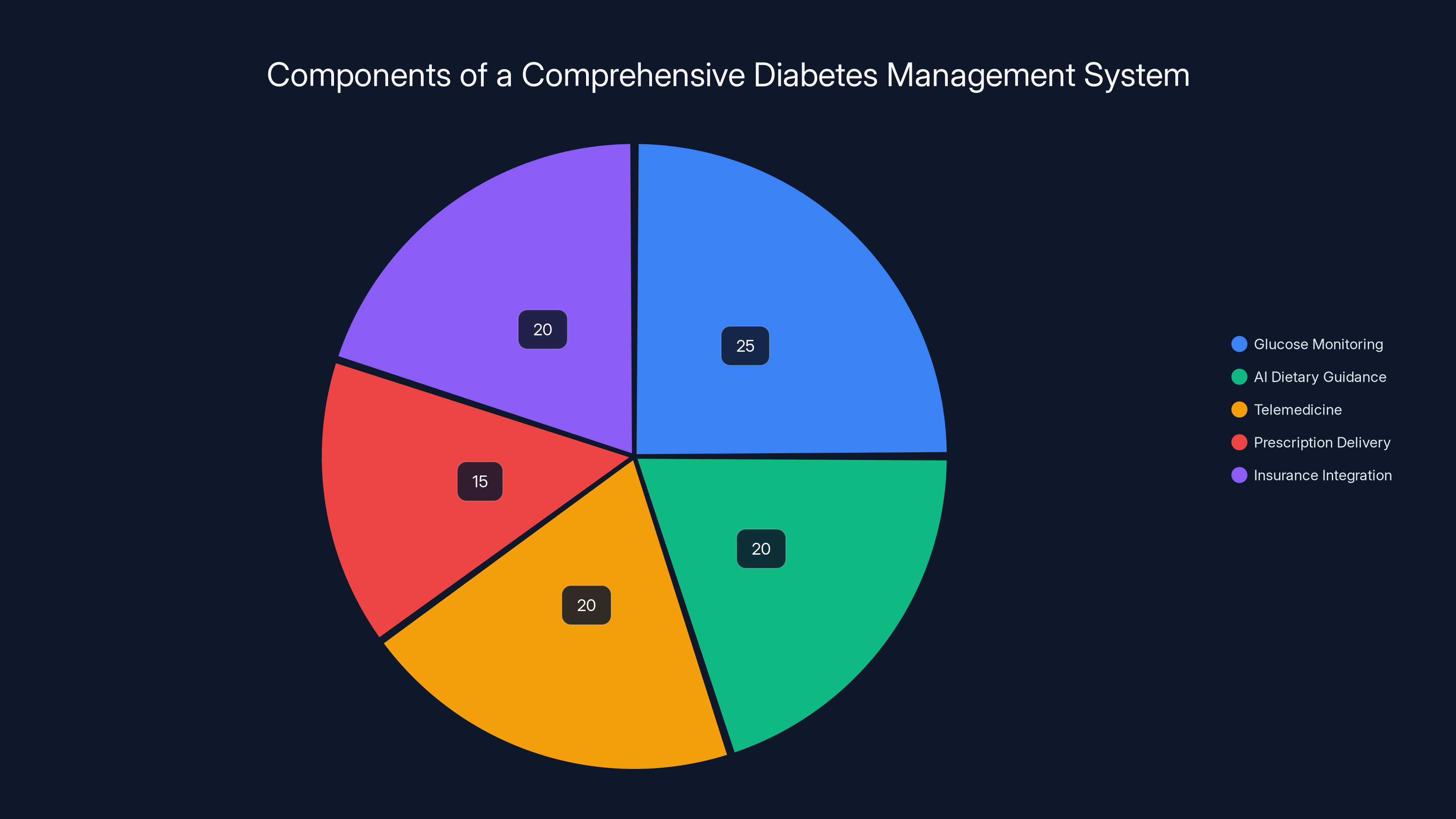
A comprehensive diabetes management system balances glucose monitoring, AI dietary guidance, telemedicine, prescription delivery, and insurance integration. Estimated data.
What Happens When Monitoring Gets Too Easy
Here's a question worth pondering: when glucose monitoring requires no friction whatsoever, what happens to adherence and behavior change?
Currently, the effort involved in glucose monitoring creates a kind of forced discipline. You check because you have to. In the process, you're constantly aware of how your food and activity affect your glucose. This awareness drives behavior change.
But if monitoring becomes effortless, you might get constant data without the behavioral friction that creates change. You'd know your glucose is spiking after sugar, but the lack of effort might mean you don't internalize the lesson deeply.
Alternatively, easier monitoring might lead to better outcomes because more people do it consistently. The friction-based adherence model doesn't work well for everyone. Some people respond better to frequent gentle feedback than to the forced discipline of difficult monitoring.
The answer probably depends on the individual and the interface design. If the companion app is thoughtfully designed to help you understand patterns and make better choices, then easier monitoring plus better feedback could dramatically improve outcomes. If the app just shows numbers without guidance, then easier monitoring might not change behavior at all.
This is where the ecosystem aspect matters. The device itself (Isaac) is just the foundation. The app, the AI analysis, the feedback mechanisms, the social features—these all determine whether the technology leads to better health or just better data collection.
Real-World Impact for Families
Let's ground this in actual lived experience. What does the Pre Evnt Isaac actually mean for someone's life?
Take a parent with a young diabetic child. Right now, their routine involves multiple finger sticks per day. The child cries, bleeds, gets frustrated. The parent gets stressed. The monitoring creates psychological burden that extends beyond the few seconds of the actual glucose test.
With Isaac, the routine becomes: breathe into the device a few times per day. No pain. No blood. No trauma. Just breathing. The psychological burden drops dramatically. The parent can actually increase monitoring frequency without guilt. The child might even view it as a game rather than a medical procedure.
Imagine the cascade of impacts. Better-monitored glucose leads to better control. Better control means fewer hyperglycemic episodes (high glucose causing organ damage) and fewer hypoglycemic episodes (low glucose causing confusion and risk of seizure). Fewer episodes means fewer emergency room visits. Better psychological health for the whole family.
On a practical level, the family can monitor more places, more times. At school, at soccer practice, at grandma's house. Everywhere because there's no shame or visible medical device. Just a small necklace that looks like jewelry.
For elderly people with diabetes and trembling hands, the difference is similar. Instead of struggling with lancets or managing sensor insertions, they just breathe. Independence and dignity preserved.
These might seem like small improvements. But for people living with diabetes, they're transformational.

The Larger Wearables Industry Implications
The successful development of noninvasive glucose monitoring has ripple effects throughout the wearables industry. It proves that advanced sensing and machine learning can solve problems that seemed intractable just years ago.
What other "impossible" measurements might become possible? Blood pressure monitoring from heart rate data and photoplethysmography (what smartwatches already do) is becoming more accurate. Some researchers are exploring non-invasive hemoglobin (iron in blood) measurement. Others are working on cortisol and other stress hormone detection.
The pattern is consistent. Find a biological marker that correlates with what you want to measure. Build sensors to detect the marker reliably. Train machine learning algorithms on large datasets. Validate with clinical trials. Launch to market.
As more companies succeed with this approach, we'll see an explosion of noninvasive measurements entering wearables. The devices we wear will become increasingly sophisticated health monitoring tools, moving beyond step counting and sleep tracking into actual medical measurement.
This creates both opportunities and challenges. The opportunities are obvious: better health insight, earlier disease detection, more accessible health monitoring. The challenges are regulatory (every new measurement requires FDA review), privacy (more health data to protect), and behavioral (handling the psychological impact of constant health feedback).
But the direction is clear. Wearables are evolving from fitness gadgets to serious health tools. Pre Evnt Isaac is a milestone in that evolution.
Manufacturing and Scale
Getting a device from lab to market requires solving manufacturing problems. Isaac needs to be mass-produced at scale. The sensors need to be reliable and consistent. Quality control needs to ensure every device meets specifications.
This is harder than it sounds. Sensor manufacturing is tricky. Bioelectronics manufacturing is even trickier. One small manufacturing issue can cause widespread failures that damage company reputation and regulatory approval.
Pre Evnt will need to establish manufacturing partnerships or build manufacturing capacity. They'll need supply chains for components. They'll need quality control processes. They'll need to ensure that the first million devices sold are as good as the prototypes demonstrated at CES.
The scale problem is why many promising medical devices fail to reach market. The inventors create something that works but can't figure out how to manufacture it reliably at volume. Pre Evnt will need to execute flawlessly on manufacturing to convert their technical innovation into commercial success.
Given the size and resources of the investment backing Pre Evnt, they likely have manufacturing figured out. But it's a risk factor that shouldn't be overlooked.
Competitive Threats and Market Dynamics
As word spreads about Pre Evnt's success with breath-based glucose monitoring, competitors will accelerate their own programs. Some will try to copy the approach quickly. Others will pursue different technical approaches.
The company with FDA approval first will have significant advantages. They'll establish market presence, build brand awareness, and potentially secure insurance coverage before competitors arrive. The second and third movers will have harder times breaking through.
But the glucose monitoring market is large enough for multiple winners. Current CGM companies like Dexcom and Free Style have millions of customers. There's room for new entrants if they offer something meaningfully better.
Pre Evnt's competitive advantages are the technology (breath-based measurement), the team (experienced in medical devices), the clinical data (ongoing trials), and the design (thoughtfully developed for actual users). If they execute on manufacturing and regulatory approval, they should succeed.
Their biggest vulnerability might be larger companies with deeper resources. If Apple finally launches an integrated glucose monitor in the Apple Watch, it would be a significant competitive threat. If Google brings optical glucose sensing to Pixel Watch, that's competitive pressure. If Medtronic or Abbott pivots aggressively toward noninvasive monitoring, they could leverage existing distribution to gain market share quickly.
Pre Evnt's window to establish market position before giants enter the space might be relatively small. They need to move fast while maintaining quality and safety standards. That's a difficult balance to strike.
Expected Timeline to Market
Based on publicly available information, here's a realistic timeline:
- 2025: FDA regulatory submission happens. Clinical trials continue with expanded patient populations. Manufacturing partnerships are finalized. The app moves toward final development.
- 2026: FDA approval is granted (Q1-Q2 is realistic). Limited commercial launch begins, likely through healthcare provider channels. Insurance coverage discussions begin.
- 2027: Broader commercial availability as insurance coverage expands. Manufacturing scales up to meet demand. Competitors begin launching competing products.
- 2028-2030: Market maturation. Multiple manufacturers offering noninvasive glucose monitoring. Integration with smartwatches and health platforms becomes standard.
This timeline could accelerate if everything goes smoothly. Or it could slip if regulatory questions emerge or manufacturing challenges arise. Medical device commercialization is inherently unpredictable.
But barring major setbacks, we should see noninvasive glucose monitoring available commercially within 12-24 months from CES 2025.

Building Systems for Better Diabetes Care
Ultimately, Isaac is just one piece of a larger system for managing diabetes. The device measures glucose, but the real value emerges when that glucose data connects to broader health infrastructure.
Imagine a complete diabetes management system that includes the Isaac glucose monitor, AI-powered dietary guidance, telemedicine consultations with diabetes educators, automated prescription delivery, and insurance integration. That's the vision some companies are building.
AI platforms like Runable could play a role here, helping create automated reports and dashboards that synthesize glucose data with nutrition information, activity tracking, and medication data. Imagine a system that automatically generates weekly reports showing patterns and making personalized recommendations. That's the kind of integration that transforms good data into actual behavior change.
Pre Evnt's Isaac is launching at an optimal moment. The ecosystem for digital health is mature. Healthcare providers are ready for new monitoring tools. Insurance companies are starting to cover connected health devices. Machine learning and AI are advancing rapidly. Patients are more comfortable with health technology.
Two years ago, Isaac might have struggled to find market adoption. Two years later, there might be multiple competing options. Right now is the sweet spot.
Conclusion: The End of the Needle Era
For people with diabetes, the needle-based monitoring era is finally ending. Not immediately, not for everyone, but the trajectory is clear. Over the next 5-10 years, noninvasive glucose monitoring will become the standard approach. The finger-stick meters and inserted sensors will become obsolete.
What started with a grandfather trying to help his diabetic grandson has evolved into technology that could improve the lives of hundreds of millions of people globally. The breakthrough isn't just technical. It's compassion translated into engineering.
Pre Evnt Isaac won't be the only noninvasive glucose monitor on the market. But it's the one that actually works, and it's arriving first. That matters. Being first doesn't guarantee dominance, but it's a significant advantage in establishing the category.
For anyone managing diabetes, or caring for someone with diabetes, the arrival of practical noninvasive monitoring is genuinely worth celebrating. The quality-of-life improvements are substantial. The health outcomes will improve. The psychological burden will decrease.
And it all started with a family's need and someone who refused to accept that continuous needle sticks were the only option. Sometimes, that's all it takes to change the world.
FAQ
What is noninvasive glucose monitoring?
Noninvasive glucose monitoring measures blood sugar levels without drawing blood or inserting sensors under the skin. Devices like the Pre Evnt Isaac use breath biomarkers or other non-invasive measurements to correlate with actual glucose levels, providing continuous tracking without needles or visible devices. This approach eliminates pain and fear associated with traditional glucose testing, making consistent monitoring more feasible for children, elderly people, and anyone with needle anxiety.
How does the Pre Evnt Isaac actually measure glucose?
The Isaac measures volatile organic compounds (VOCs) in your breath, particularly acetone and other biomarkers that correlate with blood glucose levels. When you breathe onto the device, sensors capture and analyze these compounds. Machine learning algorithms trained on thousands of hours of glucose data identify patterns and calculate your current blood glucose level, transmitting the measurement to a companion smartphone app via Bluetooth. The process takes seconds and requires no blood, no needles, and no skin insertion.
Why couldn't smartwatch makers add glucose monitoring earlier?
Optical glucose monitoring through smartwatch sensors proved nearly impossible because skin varies dramatically between individuals in pigmentation, thickness, blood flow, and composition. What works for one person gives inaccurate results for another. Companies like Apple spent years and millions trying optical approaches before essentially abandoning them. The Pre Evnt approach sidesteps these problems by measuring breath biomarkers instead, avoiding the optical sensing challenges entirely.
When will the Pre Evnt Isaac be available to purchase?
Clinical trials are currently underway with FDA regulatory submission expected in 2025. If approval is granted (realistic timeline is Q1-Q2 2026), commercial availability would begin shortly after, likely first through healthcare providers before expanding to direct consumer purchase. Full market availability with insurance coverage likely expands throughout 2027 and beyond.
Who benefits most from noninvasive glucose monitoring?
Young children with diabetes benefit enormously because the approach eliminates painful needle sticks and trauma associated with traditional monitoring. Elderly people with trembling hands or arthritis struggle with finger sticks and sensor insertions, making noninvasive options transformative. People with severe needle anxiety who've avoided monitoring can finally track consistently. Ultimately, anyone with diabetes (over 537 million globally) benefits from easier, less painful monitoring that increases adherence and health outcomes.
Will this technology replace current continuous glucose monitors?
Eventually, yes. Noninvasive glucose monitoring represents a generational improvement over devices requiring skin insertion or blood draws. However, the transition will take time. Current CGM manufacturers have established user bases and insurance coverage. Early noninvasive devices will coexist with traditional monitors during a transition period. But as noninvasive technology improves and gains FDA approval, market share will shift dramatically toward the easier, more convenient approach.
How accurate is breath-based glucose measurement compared to blood tests?
Pre Evnt has conducted extensive clinical trials comparing Isaac measurements with gold-standard laboratory glucose tests and continuous glucose monitor readings. The trials involve diverse patient populations including adolescents with type 1 diabetes and adults with type 2 diabetes. While specific accuracy data hasn't been publicly released pending FDA review, the approach has proven reliable enough to warrant continuation of clinical trials and advancement toward regulatory approval, suggesting strong accuracy performance.
What's the difference between the Pre Evnt Isaac and continuous glucose monitors already on the market?
Existing continuous glucose monitors like Dexcom and Free Style require inserting a sensor under your skin that stays for 10-14 days. The Isaac requires no insertion—you simply breathe into it multiple times per day. Both provide continuous glucose tracking, but Isaac is truly noninvasive while current CGMs are minimally invasive. Isaac is also smaller, wearable as jewelry, and doesn't visibly advertise the user's medical condition. The main trade-off is that Isaac requires active breathing samples rather than passive continuous measurement.
How does the Pre Evnt Isaac integrate with other health apps and smartwatches?
The Isaac's companion app currently lets users log meals and track glucose over time, with alerts to emergency contacts if glucose becomes dangerously low. Integration with broader health ecosystems is planned as the app development continues. Future integration with Apple Health, Google Fit, and fitness platforms would allow glucose data to connect with activity, sleep, nutrition, and stress data, enabling more comprehensive metabolic insights and personalized recommendations.
Is my glucose data private and secure on the Pre Evnt Isaac app?
Pre Evnt collects glucose data through the companion smartphone app, which stores readings and syncs to cloud servers. Privacy and security practices haven't been extensively detailed yet, but as the company moves toward commercial launch, comprehensive privacy policies and security standards will become critical for building user trust. The company will need to comply with HIPAA regulations in the US and similar privacy frameworks globally to protect sensitive health information.
Key Takeaways
- PreEvnt Isaac successfully measures glucose noninvasively by detecting breath biomarkers, eliminating needles and skin insertion
- FDA approval expected 2025-2026 following clinical trials at major institutions like Indiana University
- Technology transforms quality of life for children and elderly people by removing painful monitoring barriers
- Breath-based measurement sidesteps the skin variation problems that defeated Apple Watch and other optical approaches
- Market will expand beyond diabetics as optimization-focused fitness community adopts noninvasive monitoring
![Noninvasive Blood Glucose Monitors: The Wearable Breakthrough [2025]](https://tryrunable.com/blog/noninvasive-blood-glucose-monitors-the-wearable-breakthrough/image-1-1767870811567.jpg)



