FDA Rejects Moderna's mRNA Flu Vaccine: What Just Happened and Why It Matters
On February 3rd, 2025, the FDA delivered news that caught even seasoned vaccine industry observers off guard. The agency's Center for Biologics Evaluation and Research flatly refused to review Moderna's application for an mRNA influenza vaccine called mRNA-1010. This wasn't a rejection after review. This was a refusal to even look at the application, as reported by Stat News.
For a company that had invested hundreds of millions of dollars into a Phase 3 trial enrolling nearly 41,000 participants, the move stung. But for public health policy watchers, it represented something far more significant: a tangible manifestation of the anti-vaccine ideology now driving regulatory decisions at the highest levels of the federal government, as noted by CNBC.
The timing matters. We're living through an unprecedented moment where vaccine skepticism has moved from the fringes of conspiracy forums into the corridors of power. Robert F. Kennedy Jr., a prominent anti-vaccine activist, now holds a position of extraordinary influence over pharmaceutical regulation and public health policy. In just his first year in office, he's already dismantled childhood vaccine recommendations, canceled $500 million in pandemic preparedness research funding for mRNA vaccines, and is reshaping the FDA's stance toward vaccine innovation in ways that could reverberate through the coming decade, according to BioPharma Dive.
This article unpacks what happened with Moderna's vaccine, why the FDA's reasoning doesn't hold up under scrutiny, what this means for vaccine innovation in America, and what's really driving these decisions. Because this isn't about one vaccine. It's about whether the United States can remain a leader in pharmaceutical innovation when ideology has begun to override evidence-based decision-making at the regulatory level.
TL; DR
- FDA Rejected Without Review: The agency refused to review Moderna's mRNA flu vaccine (mRNA-1010) on February 3rd, 2025, citing comparator design issues despite previously approving the trial design twice, as highlighted by The Boston Globe.
- Unprecedented Move: This represents an extraordinary regulatory action—the FDA refused review entirely rather than requesting modifications or identifying safety/efficacy concerns, as reported by The Washington Post.
- RFK Jr.'s Influence: The decision aligns with Robert F. Kennedy Jr.'s anti-vaccine agenda, which has already cut pandemic research funding by $500 million and rolled back childhood vaccine recommendations, according to HHS.
- Trial Was Robust: Moderna's Phase 3 trial enrolled 41,000 participants and compared mRNA-1010 to FDA-approved Fluarix—a comparator used successfully in previous vaccine trials that gained approval, as noted by Investing News.
- Bottom Line: The FDA is applying novel regulatory standards retroactively to block an mRNA vaccine, signaling that innovation in this vaccine class is now viewed unfavorably by leadership, as discussed by Fierce Biotech.
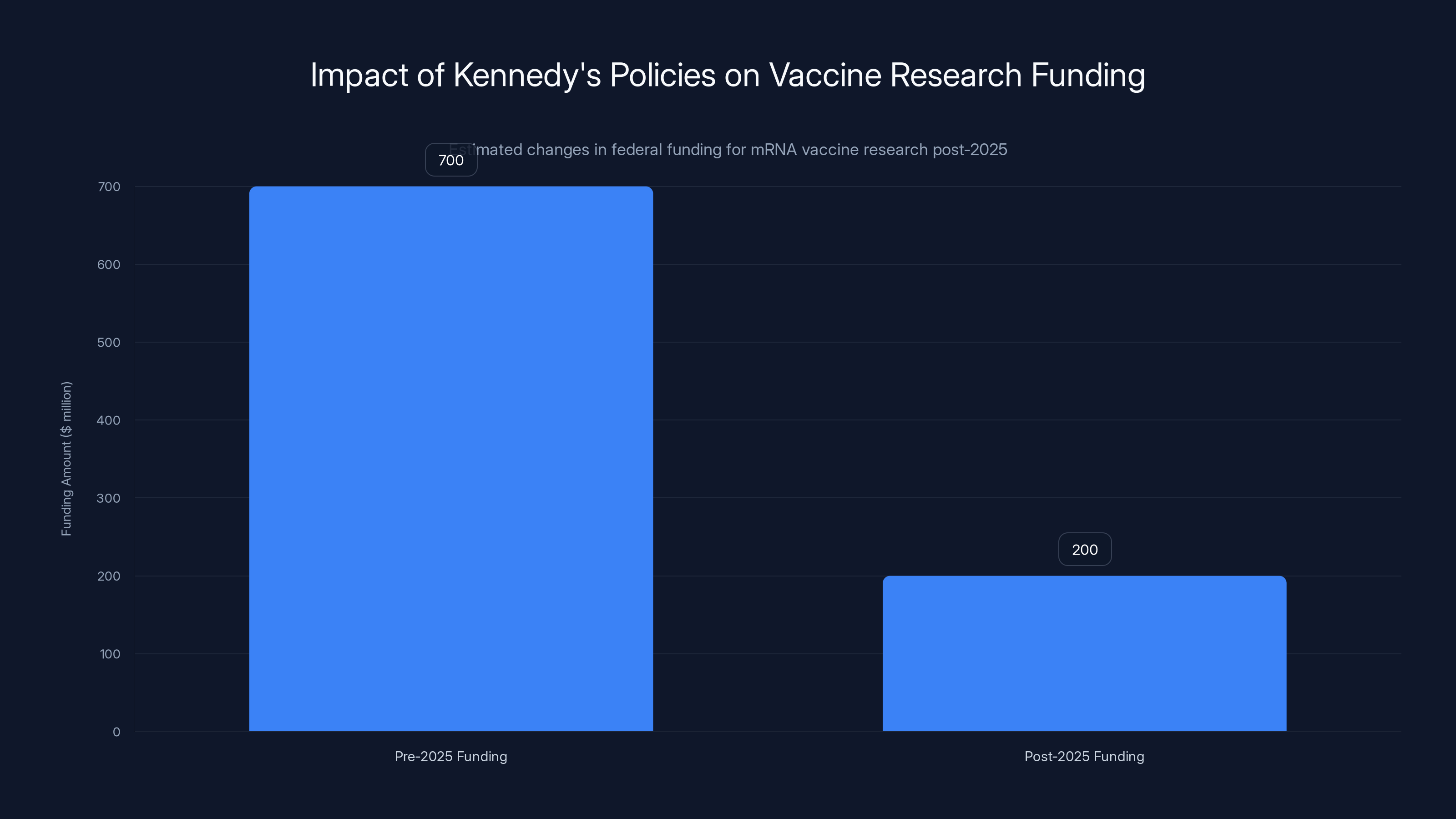
Estimated data shows a significant reduction in federal funding for mRNA vaccine research post-2025, reflecting Kennedy's policy changes.
Understanding the Regulatory Rejection: Timeline and Key Details
Moderna's mRNA flu vaccine program followed a methodical path through FDA review processes. Here's the critical timeline that reveals how extraordinary this rejection truly is.
The Trial Design Approval Process
Back in April 2024, Moderna submitted its Phase 3 trial design for mRNA-1010 to the FDA's Center for Biologics Evaluation and Research. The company proposed comparing their mRNA vaccine against two licensed, standard-dose influenza vaccines: Fluarix and Fluzone. The FDA reviewed this design and provided written approval, as documented by Stat News.
Ten months later, in August 2025, with the trial already underway and nearly 41,000 participants enrolled, Moderna submitted updated trial design information. The FDA reviewed these updates and again provided approval in writing. This is the critical detail that makes February's refusal so striking. The agency didn't say "we have concerns about your comparator"—at least, not until after they'd already told the company twice that the design was acceptable.
Moderna ran the trial exactly as approved. The company enrolled participants across multiple sites, maintained rigorous protocols, and by late 2025, had enough data to support an application for regulatory review. The trial found that mRNA-1010 was superior to the comparator vaccines in safety and efficacy measures, as highlighted by CNBC.
The Sudden About-Face
Then came the letter. Dated February 3rd, Vinay Prasad, the FDA's top vaccine regulator in the Trump administration, informed Moderna that the agency would not review the application. The stated reason: the comparator vaccine "does not reflect the best-available standard of care," as reported by The Washington Post.
This is where the regulatory logic becomes difficult to follow. First, Moderna notes that neither FDA regulations nor existing FDA guidance documents reference any requirement for a "best-available standard of care" in comparator vaccines. This isn't the company spinning the facts—this is a straightforward observation about what the actual regulatory framework requires.
Second, Fluarix and Fluzone are not obscure vaccines gathering dust in a warehouse. These are widely used, FDA-approved influenza vaccines that have been the standard of care for routine flu vaccination for years. They're given to millions of Americans annually. Glaxo Smith Kline's Fluarix has been used as a comparator in previous influenza vaccine trials—trials that resulted in approved vaccines.
So if Fluarix was adequate as a comparator in trials that led to vaccine approvals, why is it suddenly inadequate?
What "Best-Available Standard of Care" Actually Means
The best-available standard of care for influenza vaccination currently consists of these FDA-approved vaccines: Fluzone, Fluzone High-Dose (for seniors), Fluzone Quadrivalent, Fluarix, Fluarix Quadrivalent, and several others. These represent what's actually available and actually used in clinical practice.
Now, there are newer vaccines available. Shingrix (for shingles) represents a more modern approach than older shingles vaccines. For RSV, newer vaccines show superior efficacy compared to hypothetical older comparators. But for influenza? The landscape is different.
Some might argue that the highest-dose formulations should be the comparator. Others might say vaccines with newer adjuvants should be used. But these arguments are epidemiologically and scientifically reasonable positions that should be debated through proper regulatory channels, not imposed retroactively after trial completion.
What seems to have happened instead is that someone in the FDA decided, after two prior approvals of the trial design, that a different comparator would be "better." This isn't science evolving. It's the regulatory goalpost moving.
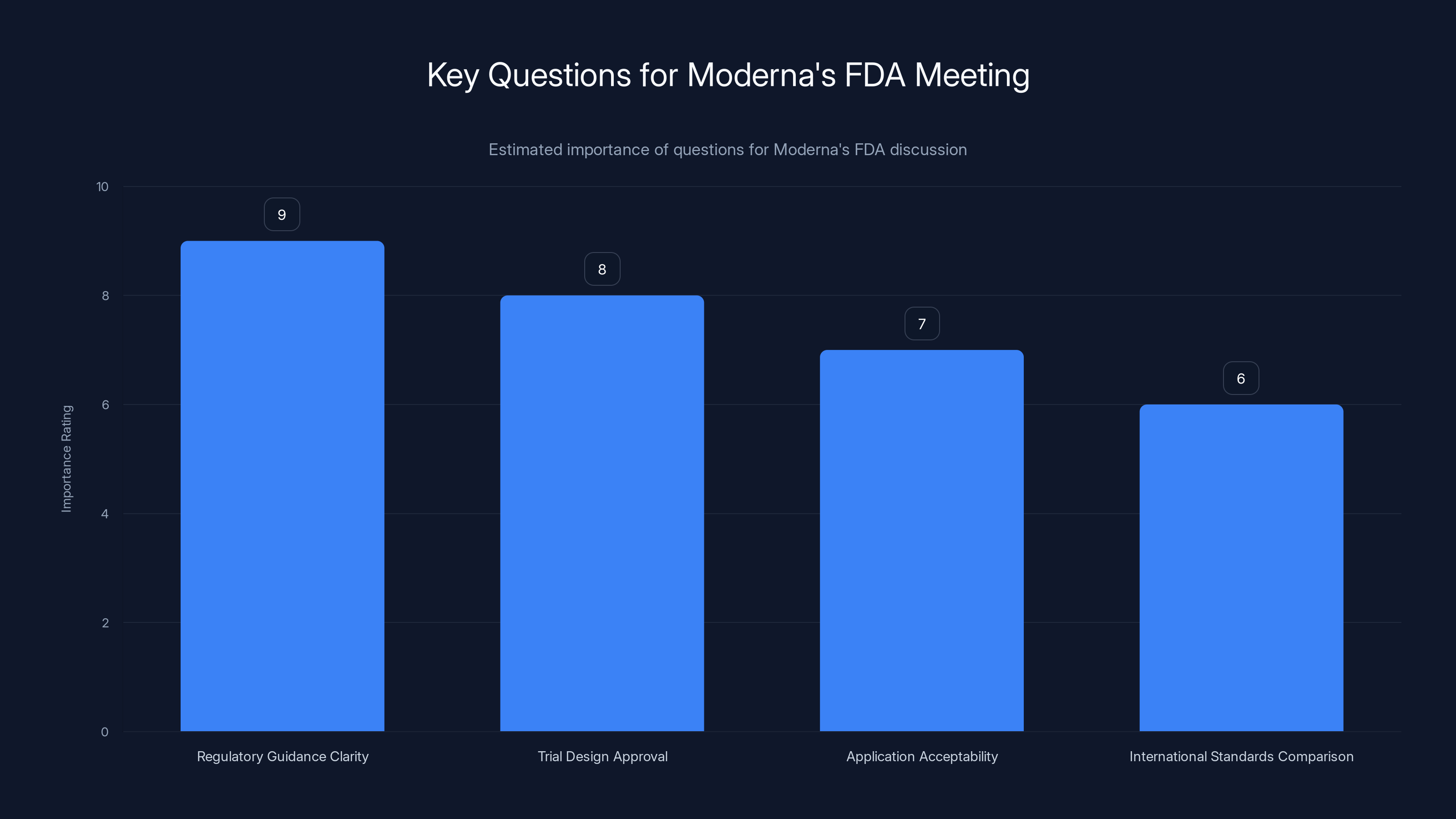
Estimated importance ratings suggest that clarity on regulatory guidance is the most critical question for Moderna's meeting with the FDA. Estimated data.
The Role of Robert F. Kennedy Jr. in Reshaping Vaccine Regulation
To understand why the FDA suddenly became more hostile to mRNA vaccine innovation, you need to understand the political context in which this decision was made.
Kennedy's Anti-Vaccine History
Robert F. Kennedy Jr. has spent decades building a platform on vaccine skepticism. He's made numerous claims about vaccine safety that have been thoroughly debunked by scientific research. He's promoted the thoroughly discredited idea that vaccines cause autism, despite the original paper supporting that claim being retracted due to serious ethical violations and fraud, as discussed by Scientific American.
For many years, Kennedy operated outside mainstream institutions. He was the voice at anti-vaccine rallies, a guest on alternative health podcasts, and a litigant filing lawsuits against pharmaceutical companies based on claims that didn't hold up in court.
Then something shifted. A political movement decided that vaccine skepticism was a useful wedge issue. Kennedy's visibility increased. He appeared on major podcasts. His book sales grew. And most significantly, in 2025, he gained direct authority over vaccine policy in the United States, as noted by HHS.
Kennedy's Impact on Pandemic Preparedness
One of Kennedy's first major acts in his position was to cancel $500 million in federal research funding for mRNA vaccines against potential pandemic threats. This wasn't a budget cut resulting from some larger fiscal pressure. This was a deliberate decision to defund a specific vaccine technology that had proven effective during COVID-19, as reported by BioPharma Dive.
Consider what that means. The mRNA platform proved it could be deployed rapidly, modified quickly for new variants, and manufactured at scale during a global emergency. The logical follow-up would be to fund research into using this platform for other pandemic threats. Influenza is an obvious candidate—seasonal flu kills 12,000 to 52,000 Americans annually, and a better vaccine would save lives.
Instead, Kennedy directed that funding away from mRNA development.
Rollback of Childhood Vaccine Recommendations
Kennedy has also dramatically altered childhood vaccine recommendations. The CDC's vaccine schedule was developed over decades by pediatricians, epidemiologists, and infectious disease experts. It's based on decades of safety data and epidemiological evidence about which diseases pose threats to children at which ages.
Kennedy has worked to reverse these recommendations, arguing that fewer vaccines for children would be better. The scientific evidence doesn't support this position. Multiple large studies across different countries have found no link between the vaccine schedule and autism or developmental problems. But the evidence has never been the obstacle for Kennedy.
The FDA Appointments
The leadership changes at the FDA matter enormously. Vinay Prasad, the official who signed the letter refusing to review Moderna's vaccine, operates within an agency that has received clear signals about what vaccine innovation projects are now viewed unfavorably.
When your leadership has publicly stated skepticism about mRNA vaccines, canceled pandemic preparedness funding for mRNA vaccines, and rolled back childhood vaccine recommendations, the message to career officials becomes clear: vaccine innovation is not the priority it once was.
The Science of Influenza Vaccines and Why mRNA Represents a Genuine Advance
To understand why blocking an mRNA flu vaccine matters, you need to understand what's wrong with current flu vaccines and what mRNA could fix.
How Current Flu Vaccines Work
Conventional flu vaccines are manufactured using a process that hasn't fundamentally changed since the 1940s. Viruses are grown in fertilized chicken eggs. Scientists inoculate eggs with flu virus strains. The virus replicates inside the eggs. Scientists then harvest and inactivate the virus, purify it, and prepare it for injection.
This egg-based manufacturing approach has profound limitations. First, the process is slow. It typically takes six months from the time the WHO recommends which flu strains should be in that year's vaccine to when doses are available for vaccination. During that six months, the circulating flu virus doesn't pause—it continues mutating.
Second, growing flu virus in eggs changes the virus slightly. The mutations that occur during egg growth can actually make the final vaccine less effective at matching the circulating strains. Studies have found that egg-adapted strains can reduce vaccine effectiveness by 10 to 40 percent in some seasons.
Third, egg-based manufacturing has inherent capacity constraints. You can only grow so much virus in so many eggs. During years when demand spikes or when manufacturing complications occur, shortages become real.
What mRNA Vaccines Offer
mRNA flu vaccines work completely differently. Instead of growing actual virus, scientists sequence the genes for the flu proteins. They synthesize the mRNA instructions for making those proteins. They package the mRNA in lipid nanoparticles that can enter cells. Your own cells then read the mRNA and produce the flu proteins, which trigger an immune response.
The advantages are substantial:
Speed: An mRNA vaccine can be manufactured in weeks, not months. If a new flu strain emerges, the manufacturing process can be updated in days. This matters enormously for pandemic preparedness.
Precision: There's no egg-adaptation drift. The vaccine contains exactly what scientists designed, not a mutated version created during manufacturing.
Scalability: Manufacturing mRNA vaccines doesn't require massive farms of chicken eggs. The process can be scaled up in existing bioreactors.
Multiplexing: A single shot could potentially contain mRNA coding for multiple flu strains, multiple viruses, or both. You could have a flu-RSV-COVID combination vaccine, for example.
Efficacy: Early data from Moderna's trial suggests that mRNA-1010 produced superior immune responses compared to conventional vaccines. This could translate to better real-world protection, as noted by Investing News.
The Broader mRNA Vaccine Pipeline
Moderna's flu vaccine is part of a larger pipeline of mRNA vaccines in development. The company is working on vaccines for RSV, combination flu-RSV vaccines, and other candidates. These represent a potential transformation in how we approach viral diseases.
But that transformation only happens if the regulatory environment permits it to happen.
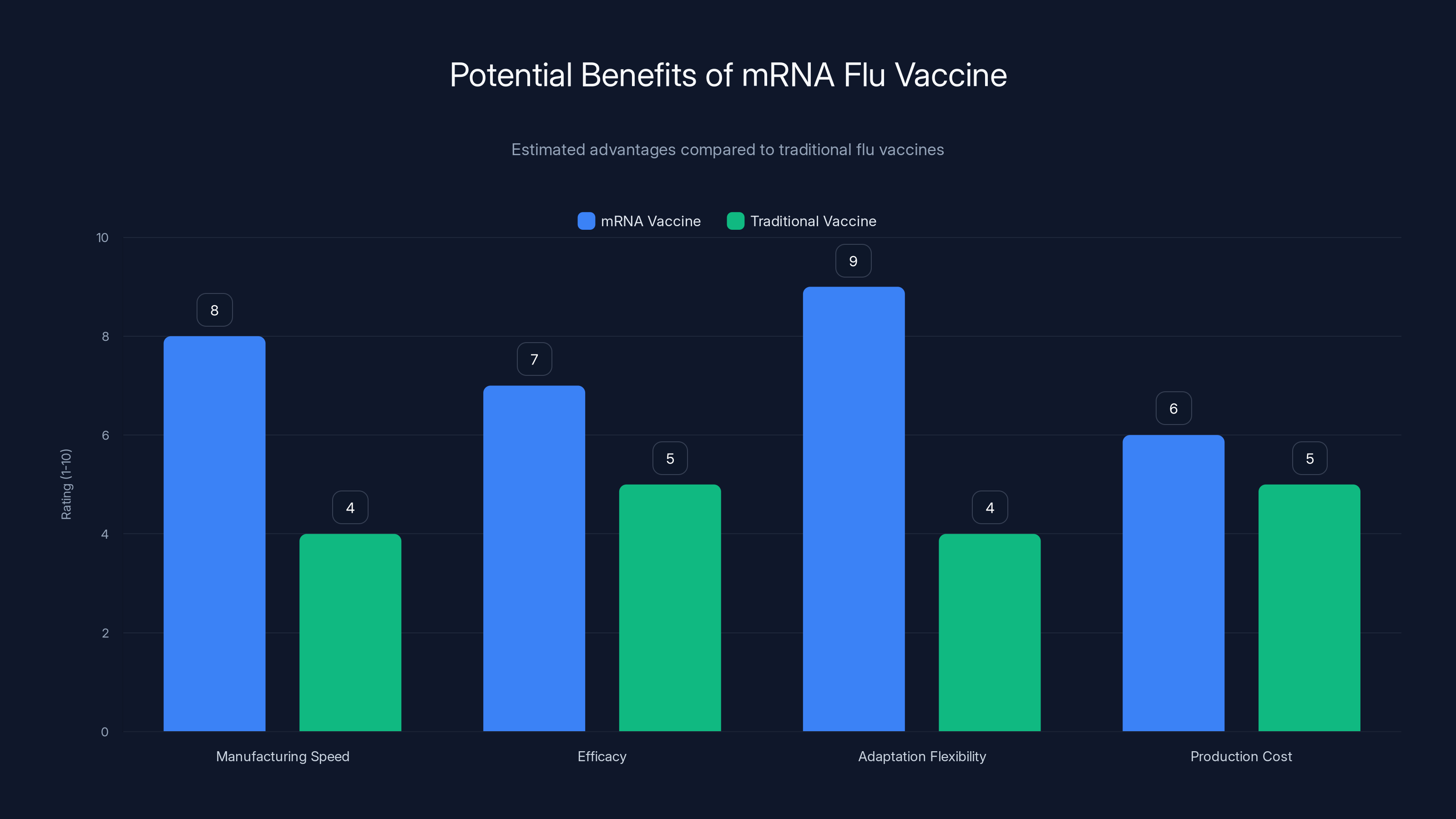
Estimated data suggests mRNA flu vaccines could significantly outperform traditional vaccines in terms of manufacturing speed and adaptation flexibility, potentially leading to better efficacy and lower production costs.
Comparator Vaccines in Clinical Trials: The Regulatory Standard
The FDA's stated objection—that Fluarix doesn't represent the "best-available standard of care"—deserves scrutiny because it reveals something about how regulatory standards are being applied.
How Comparators Work in Vaccine Trials
When testing a new vaccine, you need a control group. The ethical standard for vaccine trials is that the control group receives whatever vaccine the medical community considers standard care—not placebo. You don't withhold treatment from people who need protection.
So you divide participants into two groups. One group receives the new vaccine being tested. The other receives the standard vaccine. Then you measure which vaccine performs better.
The comparator vaccine serves multiple purposes. It ensures the trial population actually receives protection against the disease. It provides a baseline for efficacy comparisons. It allows regulators to see not just that the new vaccine works, but how much better it works than what's currently available.
Historical Use of Fluarix as a Comparator
Fluarix has a long history as a comparator in successful vaccine trials. When pharmaceutical companies tested newer flu vaccines, they compared them against Fluarix. Those trials supported regulatory approvals.
If Fluarix was adequate as a comparator three years ago, five years ago, or ten years ago, what changed? The vaccine hasn't significantly changed. The manufacturing process hasn't changed. It's still the same FDA-approved vaccine.
The only thing that has changed is the FDA's willingness to move forward with mRNA vaccine approvals.
The Best-Available Standard Problem
Here's where the regulatory logic becomes concerning. If you interpret "best-available standard of care" in the most literal sense, you might argue that the highest-dose flu vaccine should be the comparator. Or the newest vaccine with the most advanced adjuvant.
But carried to its logical conclusion, this creates a regulatory trap. Imagine a company develops a vaccine that's superior to the current best standard. They compare it against the best standard in their trial. They show superiority. But then the FDA says, "Well, your comparison vaccine wasn't the best available when we decided to review this, so we're rejecting it."
At what point is a comparator good enough? The regulation should establish this clearly in advance, not change it retroactively.
International Standards
Other regulatory bodies have looked at Moderna's trial design and deemed it acceptable. The European Medicines Agency is reviewing the vaccine. Health Canada is reviewing it. The Therapeutic Goods Administration in Australia is reviewing it. None of these agencies have objected to the comparator choice or indicated that they would refuse review based on this issue, as reported by Fierce Biotech.
This suggests that the FDA's objection is not based on an internationally recognized regulatory principle, but rather a position specific to the current FDA leadership.

Moderna's Statement and the Company's Response
The Surprise Factor
Moderna CEO Stéphane Bancel made clear in the company's statement that the refusal caught the organization completely off guard. From the company's perspective, they had done everything right. They designed a trial in consultation with the FDA. The FDA approved the design in writing. They executed the trial properly. They generated positive efficacy and safety data.
Then the agency changed the rules.
Bancel's statement captures the frustration precisely: "This decision by [the FDA's Center for Biologics Evaluation and Research], which did not identify any safety or efficacy concerns with our product, does not further our shared goal of enhancing America's leadership in developing innovative medicines," as noted by The Boston Globe.
Notice what's important here. The FDA didn't say the vaccine wasn't safe. It didn't say the vaccine wasn't effective. It didn't say the trial was flawed. It simply refused to review the application based on a requirement that appears nowhere in FDA regulations or guidance.
The Company's Regulatory Path Forward
Moderna has requested a meeting with the FDA to understand the basis for the refusal and to discuss next steps. The company is also noting that regulatory approvals in Europe, Canada, and Australia remain on track, as reported by Stat News.
This creates an interesting dynamic. If the vaccine is approved internationally but not in the United States, it raises questions about whether the FDA's decision was motivated by regulatory science or political considerations. The vaccine might eventually be approved somewhere else, where American patients would learn that their own regulatory agency rejected it for reasons other agencies found unacceptable.
The Message to the Industry
Beyond Moderna, the FDA's decision sends a message to the entire vaccine industry. If the agency can retroactively change comparator requirements after approving a trial design, no company's future investments in vaccine development are secure. This creates a chilling effect on innovation.
Why would a company invest hundreds of millions in vaccine development if the regulatory framework can shift unexpectedly? Why would investors fund vaccine startups if the FDA might suddenly decide, mid-trial, that the comparator wasn't the right one?
Innovation requires regulatory certainty. The FDA's decision undermines that certainty.

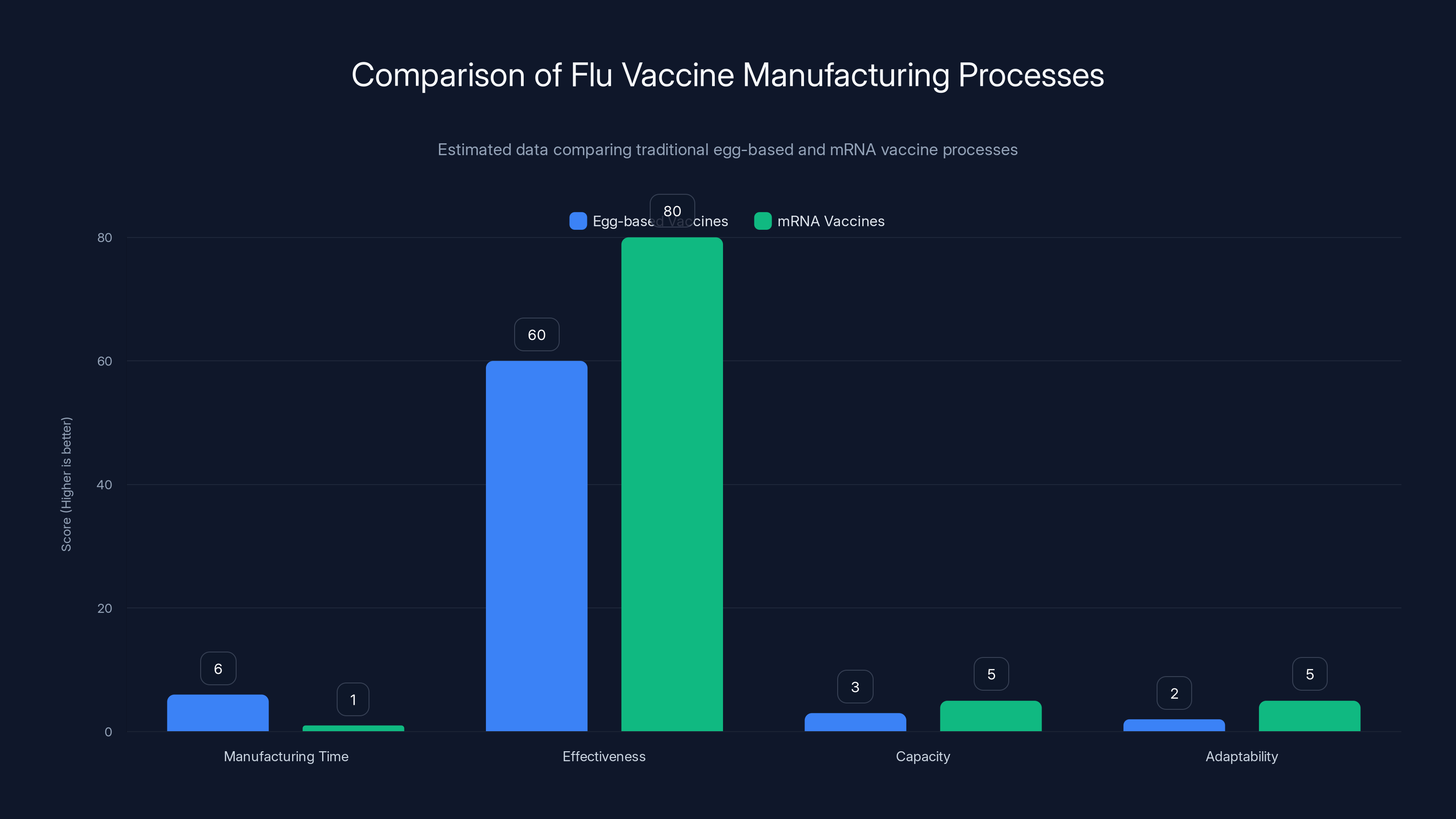
Estimated data shows mRNA vaccines offer faster manufacturing, higher effectiveness, greater capacity, and better adaptability compared to traditional egg-based vaccines.
The Broader Context: Anti-Vaccine Ideology Meets Regulatory Power
To understand why this single vaccine rejection matters, you need to zoom out and see the pattern of anti-vaccine policy decisions happening simultaneously.
Childhood Vaccine Schedule Rollbacks
The CDC's childhood vaccine schedule represents decades of evidence-based decision-making. It's been refined through countless epidemiological studies showing which diseases pose threats to children at which ages, when immunity can be optimally generated, and what sequence of vaccines produces the best protection.
Kennedy has worked to roll back recommendations from this schedule. The argument is typically framed as "giving parents a choice" or "letting people decide their own vaccination status."
But here's the epidemiological reality: vaccine-preventable diseases were common killers of children. Measles caused thousands of childhood deaths annually in the pre-vaccine era. Polio paralyzed thousands. Diphtheria, pertussis, and tetanus killed infants and children regularly.
The vaccine schedule is designed to prevent these deaths. Children who skip vaccines aren't just risking their own health—they're creating reservoirs for disease transmission to infants too young to be vaccinated and immunocompromised people who can't safely receive vaccines.
Research Funding Cuts
The $500 million cut to mRNA pandemic preparedness research represents a loss of future capacity to respond to emerging threats. This wasn't a difficult choice to make based on evidence. This was an ideologically driven decision to defund a vaccine technology.
MRNA platforms are being studied for malaria, tuberculosis, influenza, RSV, and potential pandemic threats like avian flu. The research that would have been funded by that $500 million was aimed at developing the capability to quickly modify vaccines for new pathogens.
Cutting this research doesn't eliminate future pandemic threats. It just means the United States will be less prepared when a novel pathogen emerges.
The Pattern
When you step back and look at the cumulative effect of these decisions—rolling back childhood vaccine recommendations, cutting pandemic research funding, refusing to review an mRNA vaccine application, signaling skepticism about vaccine innovation—you're seeing a coordinated effort to reshape vaccine policy based on ideology rather than evidence.
This would be concerning even if the ideology were sound. But the evidence is overwhelming that vaccines are among the most effective public health tools ever developed. Rolling back vaccine recommendations doesn't make Americans safer. It makes them less safe.
What This Means for Vaccine Innovation and American Leadership
Beyond the specific case of Moderna's flu vaccine, this decision has implications for America's position in vaccine innovation globally.
The Innovation Pipeline Risk
Vaccine development is a capital-intensive, time-consuming process. Companies need to invest billions and wait years before seeing any return. The regulatory pathway must be clear and predictable for this investment to make sense.
When the FDA changes requirements mid-trial, it makes the entire enterprise riskier. Companies become less willing to make large capital investments. Smaller vaccine companies, which might develop specialized vaccines for niche populations, may find it impossible to navigate unpredictable regulatory requirements.
This doesn't benefit public health. It harms it.
Global Competition
China and Russia are investing heavily in vaccine development. The EU has created regulatory frameworks that have attracted vaccine companies. Japan has become a hub for vaccine manufacturing and development.
If the United States becomes a place where vaccine innovation is viewed skeptically by regulatory authorities, companies will develop vaccines elsewhere. Americans will still be vaccinated—but with vaccines developed in other countries, manufactured in other countries, and generating economic value in other countries.
The vaccines work the same. The geopolitical implications are different.
Manufacturing Capacity
The mRNA vaccine platforms developed during COVID-19 created manufacturing capacity in the United States. That capacity could be used for other vaccines, other diseases, other applications. If mRNA vaccines aren't developed and approved, that capacity sits idle or gets repurposed abroad.
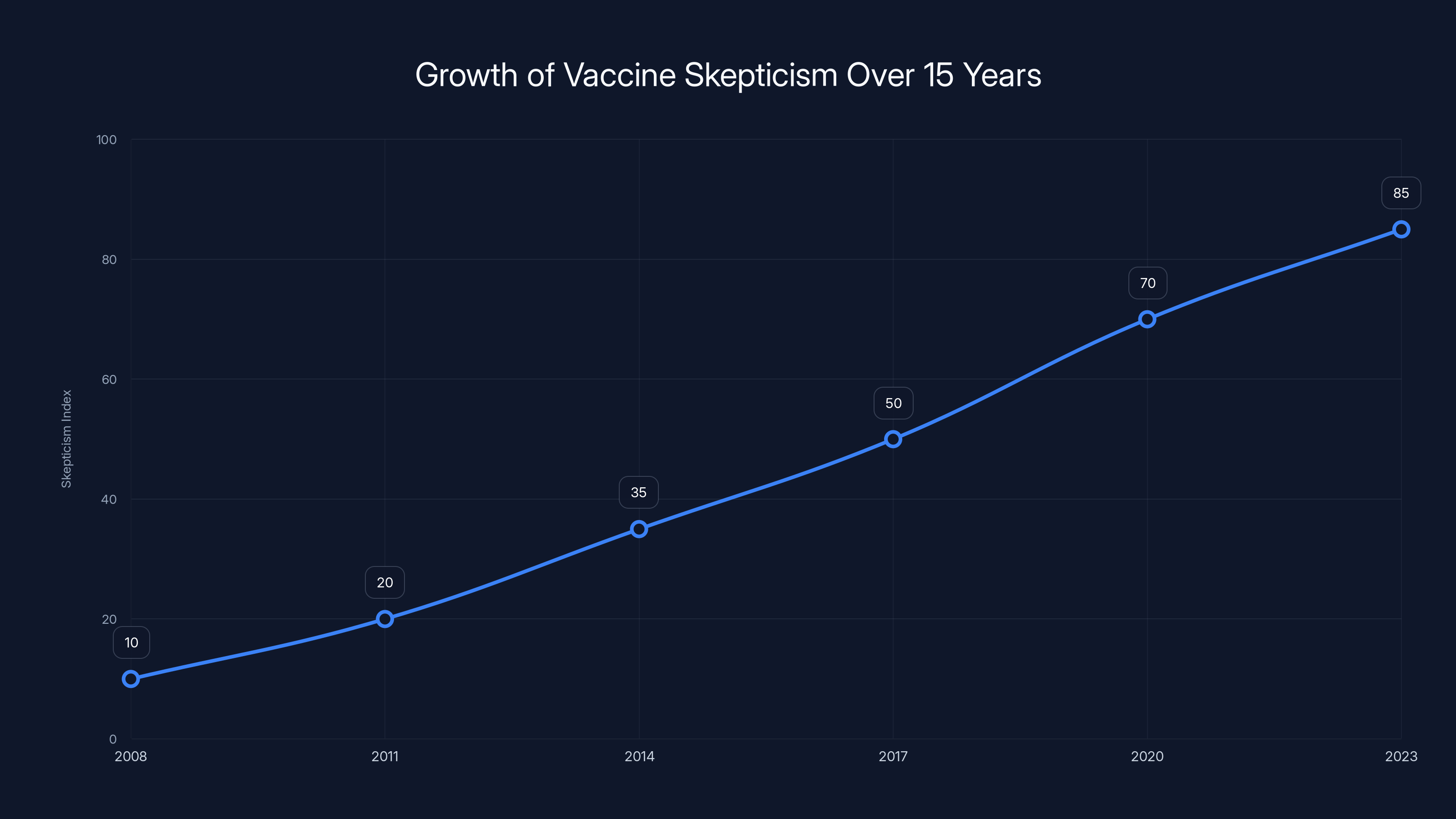
Estimated data shows a significant rise in vaccine skepticism over the past 15 years, highlighting the growing influence of anti-vaccine activism.
How Other Regulatory Bodies Are Responding
European Medicines Agency
The EMA has accepted Moderna's mRNA-1010 application for review. The European regulatory body didn't have a problem with the comparator vaccine. They didn't require changes to the trial design. They're proceeding with evaluation, as reported by Fierce Biotech.
This isn't because European regulators are more lenient. The EMA is actually quite rigorous. It's because the comparator Moderna chose is, in fact, appropriate by international standards.
Health Canada
Health Canada is also reviewing the vaccine. Canada's regulatory process is similarly rigorous to the FDA's. The fact that Canada didn't object to the trial design suggests the FDA's objection is an outlier, as noted by Stat News.
Australian TGA
The Therapeutic Goods Administration in Australia is proceeding with review. Australia has experienced serious flu seasons—in 2022, influenza vaccination rates were lower than desired and illness burden was significant. A more effective flu vaccine would be valuable in that market.
The TGA's acceptance of the application further suggests that international regulatory practice doesn't align with the FDA's position.
The Implication
When the FDA takes a position that other major regulatory bodies find unacceptable, it raises questions about whether the decision is based on regulatory science or something else. In this case, it's difficult to argue that the decision is grounded in shared international regulatory principles.

The Scientific Case for Better Flu Vaccines
Beyond the regulatory dispute, there's a genuine scientific case for developing better influenza vaccines.
Current Vaccine Effectiveness Limitations
Influenza vaccines typically provide 40 to 60 percent protection against symptomatic infection in years when the vaccine is well-matched to circulating strains. In years when there's a poor match, effectiveness drops below 30 percent.
Compare this to vaccines for other diseases. Measles vaccines are 97 percent effective. Polio vaccines are 99 percent effective. Diphtheria, tetanus, and pertussis vaccines are 95 to 98 percent effective.
Influenza vaccines work, but they're not dramatically effective. They're much better than nothing, but they leave room for improvement.
Why mRNA Could Improve This
mRNA vaccines offer several potential advantages that could improve flu vaccine effectiveness.
First, the manufacturing precision could lead to higher-quality antigens that generate better immune responses. Second, the speed of manufacturing could allow for better strain selection and faster response to emerging variants. Third, the mRNA platform allows for polyvalent vaccines—multiple flu strains and potentially multiple viruses in a single injection.
Early trials suggest these advantages are real. Moderna's data showed superior immune responses with mRNA-1010 compared to conventional vaccines, as noted by Investing News.
The Public Health Benefit
Influenza kills thousands of Americans annually. Most of these deaths occur in people over 65 or those with chronic health conditions. A vaccine that's 70 or 75 percent effective instead of 50 percent effective would prevent hundreds or thousands of deaths annually.
From a public health perspective, this matters. The resources don't exist to argue against an innovation that could prevent thousands of deaths.
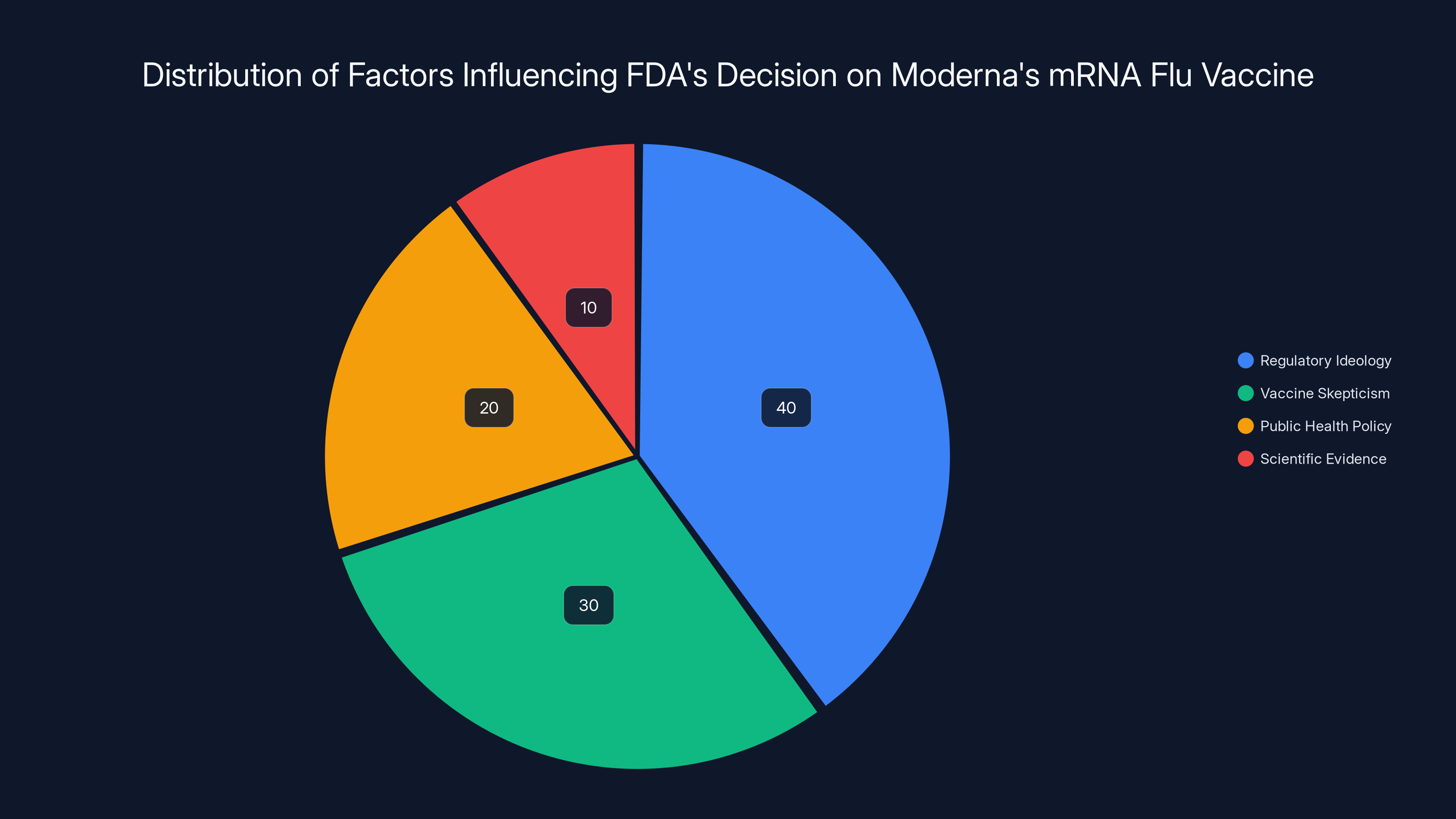
The pie chart illustrates the estimated influence of different factors on the FDA's decision to refuse Moderna's mRNA flu vaccine application. Regulatory ideology and vaccine skepticism appear to be the dominant influences, overshadowing scientific evidence.
Timeline: How We Got Here
Pre-2024: Development and Preclinical Work
Moderna began developing mRNA-1010 years before the FDA's recent actions. The vaccine had been in development for roughly five years when the company initiated its Phase 3 trial.
April 2024: Initial Trial Design Approval
Moderna submitted its Phase 3 trial design, including the choice of Fluarix and Fluzone as comparator vaccines. The FDA reviewed the design and provided written approval, as documented by Stat News.
2024-2025: Trial Enrollment and Execution
The Phase 3 trial enrolled nearly 41,000 participants across multiple sites. The trial was conducted according to the FDA-approved protocol. Participants were assigned to receive either mRNA-1010 or a standard comparator vaccine, and efficacy and safety data were collected over time.
August 2025: Updated Trial Design Review
With the trial already well-underway and substantial data accumulating, Moderna submitted additional trial design information and efficacy interim data to the FDA. The FDA reviewed these updates and provided written approval.
Late 2024 to Early 2025: Trial Completion and Analysis
The trial accumulated sufficient data to support conclusions about efficacy and safety. Moderna's analysis indicated that mRNA-1010 was superior to comparator vaccines in efficacy measures and had a favorable safety profile.
February 2025: Refusal to Review
Moderna submitted its Biologics License Application (BLA) for mRNA-1010. Vinay Prasad, the FDA's top vaccine regulator, issued a letter refusing to review the application, citing concerns about the comparator vaccine, as reported by The Washington Post.
March 2025: International Reviews Continue
While the FDA declined to review the application, the European Medicines Agency, Health Canada, and the Australian TGA all accepted the application for review. None of these regulatory bodies identified issues with the trial design or comparator choice, as noted by Fierce Biotech.

The Regulatory Process and What Should Happen Next
Standard FDA Process for Vaccine Applications
Normally, a vaccine application goes through several stages. First, the FDA does an administrative review to ensure all required documents are submitted. Then the agency conducts a substantive review of the safety and efficacy data. Finally, if everything is acceptable, the FDA approves the application or provides approval with conditions.
The FDA has significant authority to request additional information or identify deficiencies. But this request comes through the review process, not before it. The agency looks at the data, identifies questions, and asks the company for clarification.
Moderna's application appears to have been rejected before this process even began.
The Request for a Meeting
Moderna has asked for a meeting with the FDA to understand the basis for the refusal and to discuss how to move forward. This is the appropriate next step.
In that meeting, Moderna should ask for clarity on several points:
First, what specific regulatory guidance or statute requires a "best-available standard of care" in comparator vaccines? Point to the actual regulatory language.
Second, why was this requirement not mentioned when the trial design was approved in April 2024 and again in August 2025?
Third, what would Moderna need to do to make the application acceptable? Is there a different comparator that would be acceptable? If so, would the FDA accept a bridging trial comparing mRNA-1010 to a different standard vaccine?
Fourth, how do the FDA's current standards compare to international regulatory standards that have accepted the same trial design?
A Possible Path Forward
One option would be for Moderna to conduct a head-to-head comparison between mRNA-1010 and a newer or higher-dose flu vaccine. This would be expensive and time-consuming, but it might generate the data the FDA is asking for.
Another option would be to appeal the FDA's decision through administrative channels. Moderna could argue that the comparator was appropriate, that the trial was adequately designed, and that the FDA's retroactive change in requirements is unfair and unsupported by regulatory precedent.
A third option would be to focus on approvals elsewhere, launch the vaccine internationally, and gather additional data that might eventually convince the FDA to reconsider.

Anti-Vaccine Activism, Policy, and Public Health
To understand why the FDA's decision is so concerning, you need to understand the broader political context in which it occurred.
The Rise of Vaccine Skepticism
Vaccine skepticism has its roots in legitimate concerns about pharmaceutical regulation and informed consent. These are important topics. But vaccine skepticism has evolved far beyond these reasonable discussions.
Over the past 15 years, a movement has developed that rejects mainstream vaccine recommendations and promotes the idea that vaccine-preventable diseases are not serious or that vaccines are more dangerous than the diseases they prevent. These claims are not supported by evidence.
The Persistence of the Autism Myth
Perhaps the most consequential false claim in vaccine skepticism is the idea that vaccines cause autism. This myth originated from a fraudulent study by Andrew Wakefield, published in The Lancet in 1998. Wakefield manipulated data, violated ethical standards, and fraudulently obtained ethics approval for his research.
The study has been retracted. Wakefield lost his medical license. Multiple massive studies involving millions of children have found no link between vaccines and autism. The evidence is overwhelming and unambiguous.
Yet vaccine skeptics continue to promote this claim. Kennedy himself has repeatedly invoked the vaccine-autism link despite the complete absence of supporting evidence, as noted by Scientific American.
The Economic Incentives
Vaccine skepticism is profitable. Books promoting vaccine skepticism sell well. Podcasts featuring vaccine skeptics attract large audiences. Alternative health products sold to vaccine-hesitant families generate revenue.
Kennedy's positions on vaccines have made him a prominent figure in these markets. His book sales and speaking fees have increased as vaccine skepticism has grown.
Now that Kennedy has political power, he can directly shape vaccine policy in alignment with his previous advocacy. The incentive structure has shifted from building an audience to actually changing policy.
The Public Health Consequences
When vaccine skepticism influences policy, the consequences for public health are real. Measles outbreaks have occurred in communities with low vaccination rates. Pertussis outbreaks have sickened and killed infants. Polio, which was nearly eradicated globally, is reemerging in areas with low vaccination coverage.
These aren't hypothetical concerns. They're happening now.

What Moderna's Vaccine Means for Future Innovations
Beyond influenza, Moderna and other companies are developing mRNA vaccines for numerous diseases. Here's what's at stake.
RSV Vaccine
Respiratory syncytial virus (RSV) causes severe respiratory disease in infants and elderly people. An effective RSV vaccine has been elusive for decades. Moderna is developing an mRNA-based RSV vaccine that shows promise in clinical trials.
If the FDA becomes hostile to mRNA vaccine development, RSV vaccine innovation could stall.
Cancer Vaccines
Personalized cancer vaccines, which use mRNA to teach the immune system to recognize specific tumor mutations, are in development. These could potentially treat certain cancers more effectively than current approaches.
The regulatory pathway for cancer vaccines is different from vaccines for infectious diseases, but the underlying mRNA technology is the same. Anti-mRNA policies could affect cancer vaccine development.
Combination Vaccines
One advantage of mRNA platforms is that multiple diseases can be addressed in a single injection. Flu-RSV combinations, flu-COVID combinations, and other polyvalent vaccines are feasible with mRNA technology.
These combination vaccines could simplify vaccination schedules and improve vaccination rates. But they require the mRNA platform to be viewed favorably by regulators.
Pandemic Preparedness
The most concerning implication of cutting mRNA vaccine research funding is the impact on pandemic preparedness. When the next novel pathogen emerges—and history suggests this will happen—the United States wants to have the fastest, most effective tools available.
mRNA vaccine platforms provide those tools. Cutting research funding on these platforms reduces America's pandemic preparedness.

Comparison: How U.S. Policy Diverges from International Standards
The FDA's approach to mRNA vaccines diverges significantly from how other countries are managing vaccine innovation.
European Approach
The European Union has been supportive of mRNA vaccine development. The EMA has actively worked with companies to speed up vaccine development. When COVID-19 vaccines became available, European regulators approved them quickly while maintaining rigorous safety standards.
Now, with mRNA vaccines for other diseases in development, European regulators are taking these applications seriously and moving them through their review process.
Asian Markets
Japan and South Korea have also embraced vaccine innovation, including mRNA platforms. These countries recognize that vaccine development is a strategic advantage in global health.
U.S. Divergence
The United States, historically the leader in pharmaceutical innovation and vaccine development, is now moving in the opposite direction. The FDA's hostility toward mRNA vaccines represents a shift away from the innovation-friendly approach that made America a leader in these fields.

FAQ
What is mRNA-1010 and Why Does It Matter?
mRNA-1010 is Moderna's investigational messenger RNA vaccine for influenza. It differs from traditional flu vaccines by using genetic instructions rather than grown virus particles. The vaccine works by teaching cells to produce flu antigens, triggering an immune response. It matters because it represents a potential technological leap in flu vaccine efficacy and manufacturing speed, offering the possibility of better protection against seasonal and pandemic influenza strains.
Why Did the FDA Refuse to Review Moderna's Vaccine Application?
The FDA cited concerns about the trial's comparator vaccine—the standard influenza vaccine that participants in the control group received. The agency claimed the comparator did not reflect the "best-available standard of care." However, this language doesn't appear in FDA regulations or guidance, and the FDA had previously approved the same trial design in writing twice. The stated reason appears insufficient given that international regulators accepted the same trial design without objection, suggesting political rather than scientific factors influenced the decision, as noted by Fierce Biotech.
How Does Robert F. Kennedy Jr. Influence FDA Decisions on Vaccines?
Kennedy holds a position that gives him significant authority over vaccine policy and pharmaceutical regulation. He has publicly promoted vaccine skepticism for decades and has already cut pandemic preparedness funding by $500 million and rolled back childhood vaccine recommendations. When leadership expresses skepticism about vaccines generally and mRNA vaccines specifically, career officials at the FDA understand what policy priorities the leadership favors, which can influence regulatory decisions like the refusal to review Moderna's vaccine.
What Are the Potential Benefits of an mRNA Flu Vaccine?
An mRNA flu vaccine could offer several advantages: faster manufacturing (weeks instead of months), elimination of egg-adaptation drift that reduces effectiveness, better immune responses, and the potential for polyvalent vaccines combining protection against multiple viruses. These advantages could result in higher vaccine effectiveness, faster response to emerging variants, and potentially hundreds or thousands of prevented deaths annually in the United States alone.
How Do Other Countries' Regulatory Bodies View Moderna's Vaccine?
The European Medicines Agency, Health Canada, and the Australian Therapeutic Goods Administration have all accepted Moderna's vaccine application for review. None of these major regulatory bodies identified issues with the trial design or the choice of comparator vaccine. Their acceptance of the application suggests that the FDA's objection represents a nation-specific policy position rather than a universally recognized regulatory principle, as reported by Stat News.
What Does This Mean for Future Vaccine Innovation in America?
The FDA's decision creates regulatory uncertainty that could discourage investment in vaccine development. If regulatory requirements can change retroactively after trial completion, companies face higher risk and uncertainty. This could slow vaccine innovation in the United States, cause companies to develop vaccines in other countries instead, and ultimately reduce America's competitiveness in this critical sector while potentially reducing the nation's pandemic preparedness capacity.
Is There Scientific Evidence That Vaccines Cause Autism?
No. The original study claiming a vaccine-autism link was fraudulent and has been retracted. The researcher, Andrew Wakefield, manipulated data and lost his medical license. Multiple large-scale studies involving millions of children have found no link between vaccines and autism. The scientific evidence is overwhelming and definitive that vaccines do not cause autism. Vaccine skeptics continue to promote this claim despite complete lack of supporting evidence, as highlighted by Scientific American.
What Are the Real-World Consequences of Declining Vaccine Rates?
Historically vaccine-preventable diseases are resurging in areas with low vaccination rates. Measles, which was eliminated from the United States in 2000, has returned with hundreds of cases annually in recent years. Pertussis outbreaks have sickened and killed infants. These diseases kill children. Vaccination rates affect not only individual protection but community immunity, protecting infants and immunocompromised individuals who cannot be vaccinated.

The Bigger Picture: Why This Decision Represents a Critical Turning Point
Moderna's rejected vaccine application matters on its own terms. But it also represents something larger: a shift in how vaccine innovation is treated by American regulatory authorities.
For decades, the FDA has been the gold standard for pharmaceutical regulation. Companies worldwide aspire to FDA approval because it signals rigorous, science-based evaluation. That reputation has been built through decades of making difficult decisions based on evidence, not ideology.
The refusal to review Moderna's mRNA flu vaccine suggests that reputation is changing. When the FDA changes regulatory requirements retroactively, ignores its own prior approvals, and applies standards that international regulators find unacceptable, it signals that something other than regulatory science is driving the decision.
This matters for vaccine innovation. It matters for pandemic preparedness. It matters for America's position as a global leader in pharmaceutical development. And most importantly, it matters for public health.
The coming years will reveal whether the FDA's approach to mRNA vaccines is an anomaly or the beginning of a new policy direction. Vaccine companies are watching. Investors are watching. International regulators are watching.
What they see will determine whether America remains a leader in vaccine innovation or whether innovation happens elsewhere. That determination will affect not only which vaccines Americans can access, but the nation's capacity to respond when the next pandemic emerges.
For now, Moderna is requesting a meeting with the FDA. The company will try to understand what the agency wants. Perhaps the two parties will reach an understanding. Perhaps the vaccine will eventually be approved. Perhaps international approvals will prove that America's skepticism was misplaced.
Or perhaps this is the beginning of a longer shift, where anti-vaccine ideology begins to shape policy in ways that ultimately weaken public health. History will judge which scenario unfolds. For now, what matters is that a promising vaccine innovation has been blocked, and the reasons for that blocking don't hold up to scrutiny.
That's concerning. And it should be.

Key Takeaways
- It's about whether the United States can remain a leader in pharmaceutical innovation when ideology has begun to override evidence-based decision-making at the regulatory level
- 2/z1QXTxFPbcGu61fiJV9Kfw--/YXBwaWQ9aGlnaGxhbmRlcjt3PTY0MDtoPTQ0Mg--/https://media
- Png)
*Estimated data shows a significant reduction in federal funding for mRNA vaccine research post-2025, reflecting Kennedy's policy changes
- Here's the critical timeline that reveals how extraordinary this rejection truly is
- This is the critical detail that makes February's refusal so striking
Related Articles
- Spring Cleaning Motivation: Budget Accessories Starting at $2 [2025]
- Fitbit's AI Health Coach Expands to iOS: What You Need to Know [2025]
- Why Chinese Flagship Phones Are Outpacing the iPhone [2025]
- FPV Drones at Winter Olympics [2025]: Stunning Footage vs. Noise Controversy
- NYT Strands Game Tips, Hints & Strategy Guide [2025]
- The OpenClaw Moment: How Autonomous AI Agents Are Transforming Enterprises [2025]
![FDA Refuses Moderna mRNA Flu Vaccine Review Amid RFK Jr. Anti-Vaccine Push [2025]](https://tryrunable.com/blog/fda-refuses-moderna-mrna-flu-vaccine-review-amid-rfk-jr-anti/image-1-1770773807937.jpg)



