Why an FDA Official's Rejection of Moderna's Flu Vaccine Actually Matters More Than You Think
In early 2025, something quietly alarming happened inside the FDA. A political appointee with no vaccine expertise overruled a team of career scientists who were ready to review Moderna's experimental mRNA flu vaccine. The vaccine itself wasn't the problem. The team of trained regulators wasn't the problem either. Instead, one person decided the company had used the wrong comparison vaccine in their clinical trial and refused to move forward. According to Stat News, this decision was made despite the FDA scientists' recommendation for approval.
This isn't just bureaucratic infighting. This is the kind of decision that ripples through an entire industry, signaling that innovation might face unpredictable obstacles regardless of scientific merit. When a company invests hundreds of millions in developing a vaccine, spends years on clinical trials, and then gets rejected not on safety or efficacy grounds but on a technical complaint about trial design, other companies notice. Venture capitalists notice. Investors notice. As reported by The New York Times, the rejection was not based on safety concerns but rather on the choice of the comparison vaccine.
The official in question is Vinay Prasad, a blood cancer specialist with no background in vaccines or immunology. He's currently leading the FDA's vaccine division under the Trump administration. What's noteworthy isn't that he disagreed with his team's assessment—that happens in government. What's remarkable is how he disagreed: by reportedly telling staff he wants to start sending surprise rejection letters that "blindside" drug developers, and dismissing concerns about whether such moves might open the FDA to litigation. This was highlighted in a report by NBC News.
This situation reveals something fundamental about how regulatory decisions get made at the highest levels of government, and it raises uncomfortable questions about expertise, oversight, and the future of vaccine innovation in the United States.
TL; DR
- Vinay Prasad, Trump's FDA vaccine regulator, single-handedly rejected Moderna's mRNA flu vaccine review despite FDA scientists recommending approval
- The rejection wasn't based on safety concerns but on disagreement about the comparison vaccine used in Moderna's Phase 3 trial with nearly 41,000 participants
- FDA career staff objected in writing: David Kaslow, a top career vaccine official, wrote a memo opposing Prasad's decision, and scientists had an hour-long meeting with Prasad laying out their concerns
- This is part of a pattern: Moderna is at least the ninth company to receive an unexpected rejection from Prasad's office, raising industry concerns about unpredictability and innovation
- The consequences are real: Industry fears about litigation and investment uncertainty could slow vaccine development, affecting future pandemic preparedness

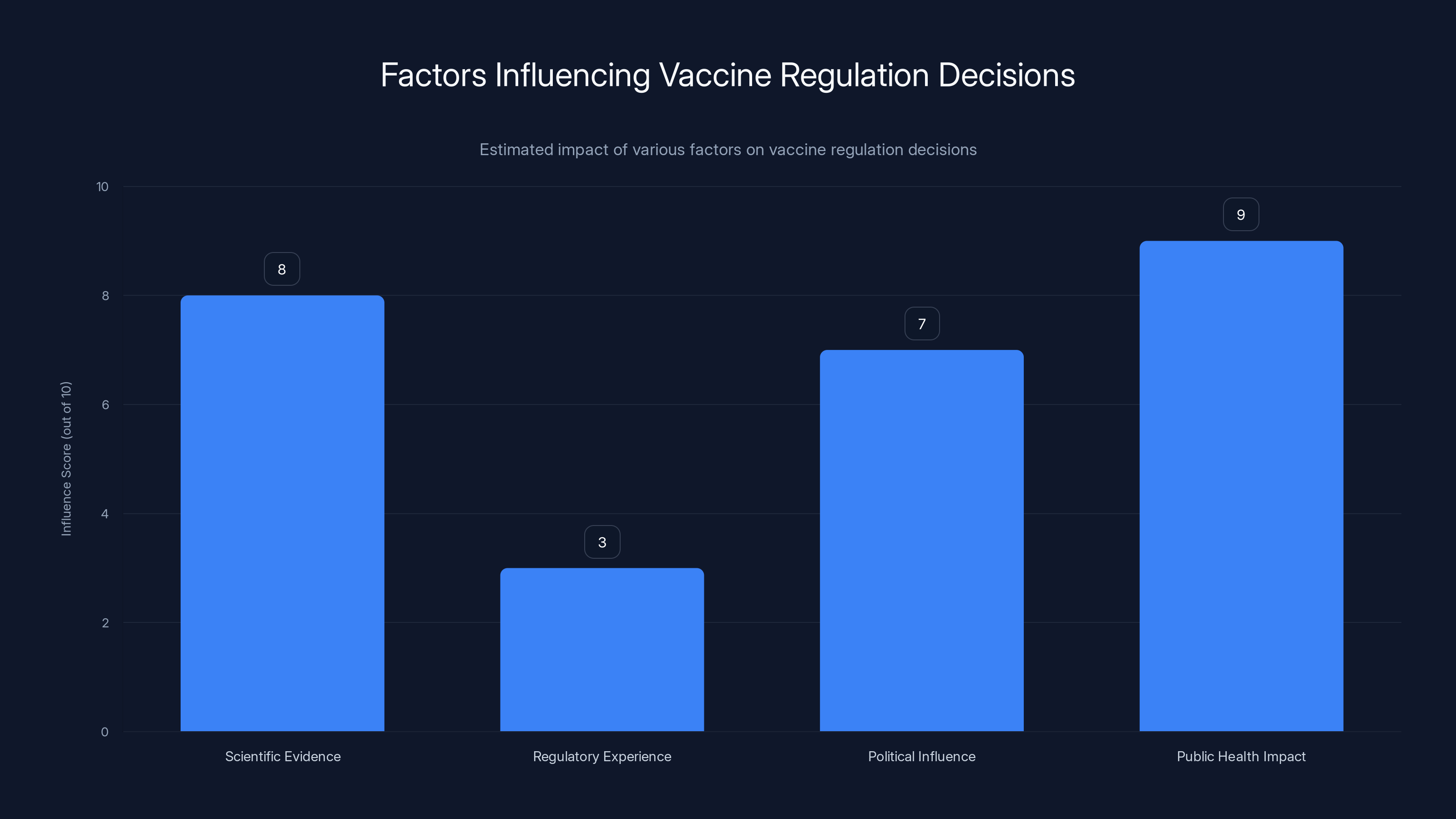
Estimated data suggests that public health impact and scientific evidence are major influences on vaccine regulation decisions, while regulatory experience may play a lesser role.
The Moderna Vaccine: What They Actually Submitted
Moderna's mRNA flu vaccine represents something genuinely novel. The company took the same technology that worked for COVID-19 vaccines and adapted it for influenza, a virus that's mutated every year for over a century. Getting flu vaccines right matters because seasonal flu kills tens of thousands of Americans annually, and current options work imperfectly—many existing vaccines hover around 40-60% effectiveness in older populations, according to the National Council on Aging.
Moderna's experimental vaccine completed a Phase 3 clinical trial that enrolled nearly 41,000 adults aged 50 and older. This is substantial. For context, many vaccines approved by the FDA come from smaller trials. The trial design wasn't invented overnight by Moderna in a vacuum. The FDA reviewed Moderna's trial plans multiple times before the company ever enrolled a single participant. During these pre-submission meetings, FDA scientists examined exactly what Moderna proposed—including the choice of comparison vaccine. The agency raised suggestions but, critically, concluded that Moderna's approach was "acceptable," as noted by Stat News.
This matters because it means the FDA signed off on the trial design prospectively. The company didn't sneak something past regulators. Scientists sat down, discussed the approach, and said yes, this works. Moderna even adapted its plans based on feedback. The FDA suggested that for participants aged 65 and older (who are eligible for high-dose flu shots), the comparison might be different. Moderna listened. They added a comparison arm using high-dose vaccine for some older participants and provided additional analysis.
When Moderna finally submitted its data, the company wasn't trying to pull something controversial. They'd followed the process. They'd incorporated feedback. They'd done what the FDA asked. Then Vinay Prasad said no, as reported by Access Newswire.
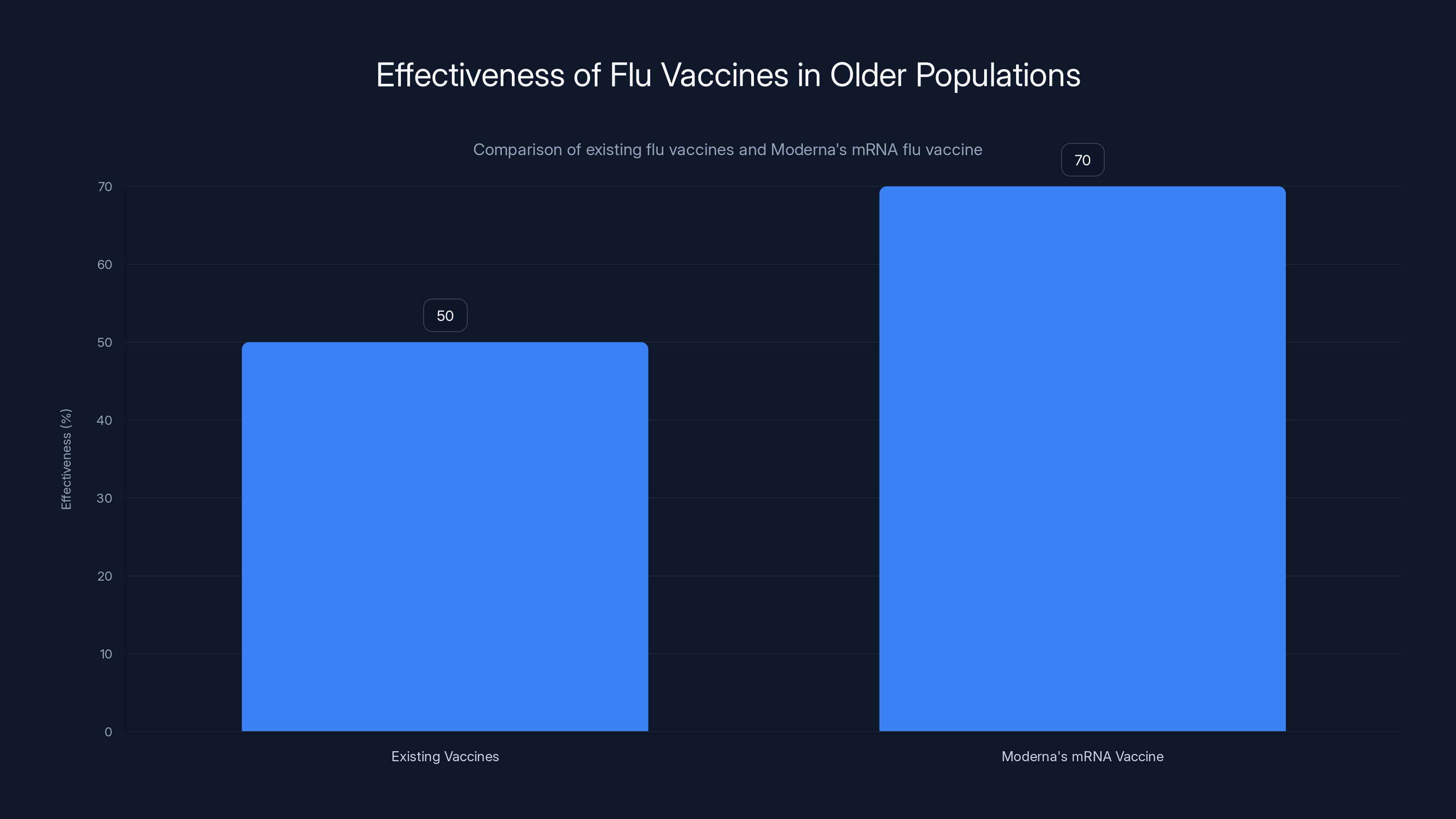
Moderna's mRNA flu vaccine shows an estimated effectiveness of 70% compared to the 40-60% range of existing vaccines in older populations. Estimated data based on trial expectations.
Who Is Vinay Prasad and Why Does His Background Matter
Vinay Prasad wasn't brought into the FDA to regulate vaccines. He's a blood cancer specialist. His training, his expertise, his career—all focused on hematologic malignancies. He's published papers on cancer drugs. He's been involved in oncology policy. He has no published research on vaccines. He hasn't spent years studying immunology. He hasn't worked inside the FDA's vaccine division before.
This isn't to say that talented people can't learn new fields. They absolutely can. But there's a difference between learning a new field and overnight becoming the top vaccine regulator at a federal agency. The FDA's vaccine division is complex. It involves immunology, epidemiology, statistical analysis of large clinical trials, manufacturing standards, and decades of institutional knowledge about how to evaluate whether vaccines are safe and effective. You don't absorb that in a few months.
More concerning is Prasad's public positioning on vaccines. Before his FDA appointment, he was known in some circles for expressing skeptical views about certain vaccines and health policies. This isn't a disqualifying characteristic—skepticism applied rigorously is valuable in science. But there's skepticism that's grounded in evidence and data, and there's skepticism that becomes ideology. The distinction matters.
Inside the FDA, people who work with Prasad describe a management style that creates what internal sources characterized as an environment "rife with mistrust and paranoia." Multiple complaints have been filed against him, including allegations involving sexual harassment, retaliation against subordinates who disagreed with him, and verbally berating staff in meetings. This context is important because it suggests Prasad doesn't just disagree with his team's science—he manages in ways that discourage people from pushing back, as detailed by BioSpace.

The Rejection That Came Out of Nowhere
Prasad's rejection wasn't subtle, and it wasn't well-telegraphed. According to reporting from Stat News and The New York Times, FDA career scientists were ready to move forward with reviewing Moderna's data. They'd planned the review. They understood the trial design. They saw no red flags that would prevent evaluation of the vaccine's efficacy and safety.
Then Prasad decided to issue a refusal letter. This isn't a minor regulatory instrument. A refusal letter tells a company their application won't be reviewed. It halts the process. It's used when there are fundamental problems—for example, if a company didn't provide required manufacturing information or if there are obvious safety issues. The FDA doesn't casually issue refusal letters.
Prasad's reasoning focused on Moderna's choice of comparison vaccine in the trial. The company used a standard-dose flu vaccine as the comparator. This is actually pretty normal. Standard-dose flu vaccines are what most Americans get. They're licensed. They work reasonably well. Comparing a new vaccine to what's already approved is a sensible study design because it shows whether you've improved on the existing standard.
But Prasad apparently wanted Moderna to have used something different. The exact nature of his objection remains somewhat unclear from public reporting, but the broad thrust was that Moderna's choice of comparator was problematic enough to warrant refusing to even look at the data. This is extraordinary. You'd typically reject a vaccine if the data showed it was unsafe or ineffective. You don't usually refuse to review data because you disagree with the study design choices that were previously deemed acceptable.
David Kaslow, a top career FDA official who reviews vaccines, wrote a detailed memo objecting to Prasad's rejection. Kaslow didn't just disagree in passing. He wrote a formal memo laying out why the review should proceed. This is a significant action—a career civil servant formally documenting disagreement with his political boss's decision, as noted by News Express KY.
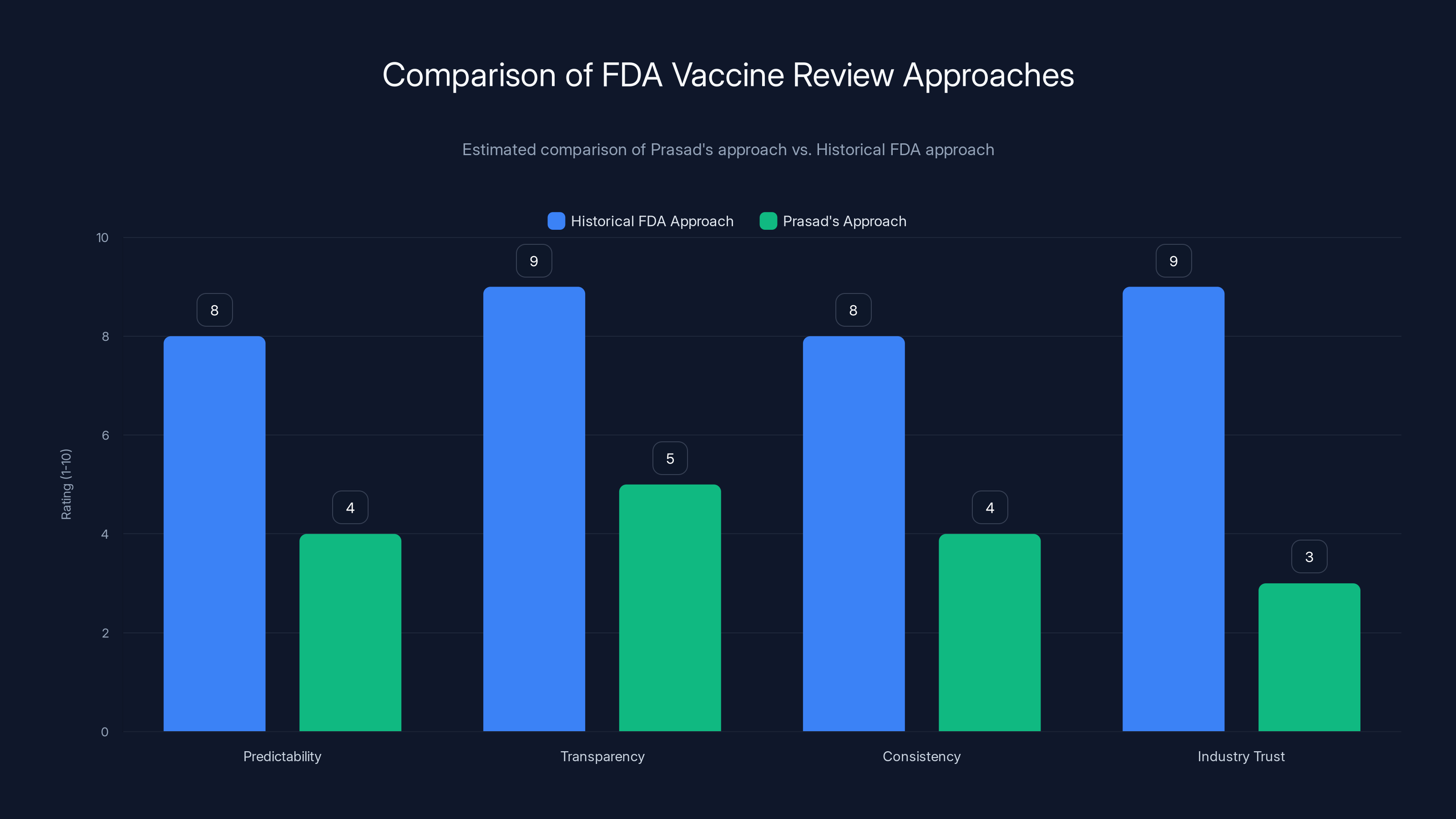
Estimated data suggests Prasad's approach scores lower on predictability, transparency, consistency, and industry trust compared to the historical FDA approach.
The Meeting Where Scientists Laid Out Their Case
In early January 2025, before the refusal letter was formally issued, FDA vaccine scientists requested a meeting with Prasad. This meeting lasted an hour. During that time, multiple career scientists presented their concerns. They explained why they believed the review should proceed. They showed him the data. They walked through the scientific reasoning.
Prasad listened. And then he apparently held firm. According to sources who spoke to the Journal, he dismissed their concerns and moved forward with his plan to refuse the review. This is the crucial moment that captures what's problematic about the situation. These weren't junior staffers or people without expertise. These were career vaccine scientists at the FDA. They had spent their careers—in some cases, decades—evaluating vaccines. They understood the science. They understood regulatory standards. They understood how to interpret clinical trial data.
And their boss, a blood cancer specialist with no vaccine background, overruled them.
It's worth asking yourself what this decision signals. If you're a company considering investing in vaccine development, what does this tell you? It tells you that even if you run the studies regulators ask for, even if you follow the process, even if you get preliminary approval for your trial design, you could still face an unexpected rejection on grounds that might be shifted on a whim.
For an industry that requires massive upfront investment and takes years to see returns, unpredictability is poison. You can't invest hundreds of millions if you don't know what the rules are. You certainly can't invest if the rules might be changed by one person after the game is already being played.
Prasad's Apparent Strategy: Surprise Rejections and Intimidation
Here's where the story gets more disturbing. According to the Journal's reporting, Prasad told FDA staff that he wants to send more refusal letters that "blindside" drug developers. That's the actual word used. Blindside. As in, surprise them with rejections they weren't expecting.
This suggests that rejecting Moderna wasn't an isolated decision based on specific concerns about that vaccine. It's part of a broader approach—a strategy to keep companies off-balance, to make them uncertain, to demonstrate that Prasad is making unpredictable decisions.
This is terrible regulatory policy. Let's be clear about that. Regulators should be predictable. Companies should know what's expected. The process should be transparent. The FDA has developed these norms over decades because they actually work better than the alternative. When regulators are unpredictable, when they move goalposts, when they use surprise rejections as a tool, it doesn't make them more effective. It makes them worse. Companies stop taking risks. Innovation slows. Development of new treatments stalls.
For vaccines specifically, this is genuinely dangerous. We live in a world where pandemic threats are real. COVID-19 showed us that. Monkeypox showed us that. The influenza virus continues to evolve in unpredictable ways. We need companies developing new vaccines. We need them investing. We need them taking risks. When the regulatory environment becomes hostile and unpredictable, those companies do less, not more.
The FDA's review staff apparently understood this. According to the Journal, they pushed back on Prasad's plans, noting that deliberately blindsiding companies breaks with FDA practices and could expose the agency to litigation. Companies that get surprise rejections might sue. They might argue the decision was arbitrary. They might claim the FDA violated its own procedures.
Prasad reportedly dismissed this concern. He wasn't worried about litigation. He apparently didn't care that his approach diverged from established practices. He just wanted to keep sending surprise rejections.

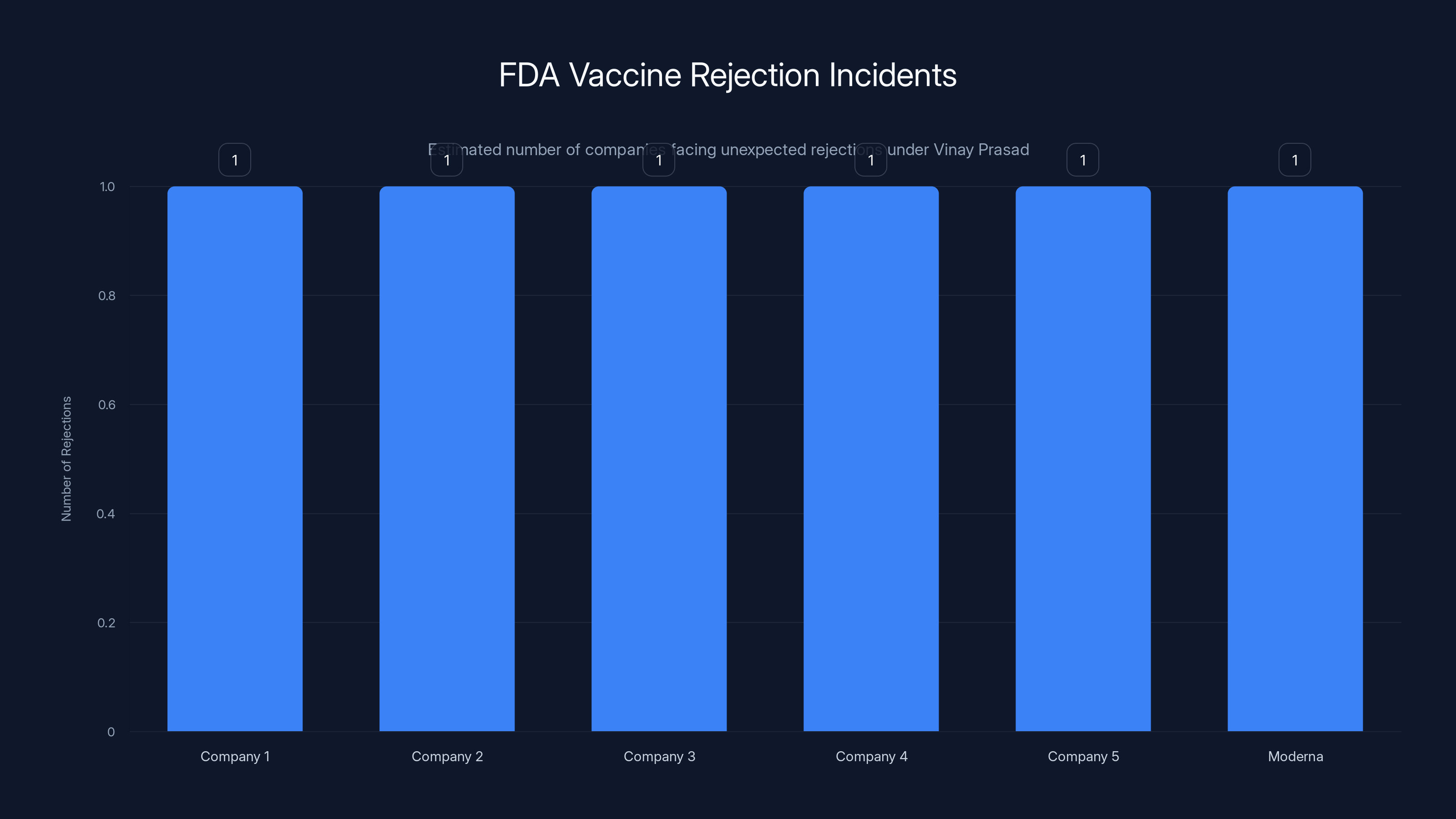
Moderna is one of at least nine companies to face unexpected rejections from Vinay Prasad's office, highlighting concerns about regulatory unpredictability. Estimated data.
The Commissioner's Contribution: Calling the Trial "Unethical"
Prasad isn't acting alone. Trump's FDA Commissioner, Marty Makary, added his voice. Makary suggested on Fox News that Moderna's trial might be "unethical." This is a remarkable claim. What's unethical about running a 41,000-person trial comparing a new vaccine to an approved vaccine?
Makary, like Prasad, doesn't have a background in vaccine development or immunology. He's a surgeon. He has experience in healthcare policy and healthcare administration. But calling a massive clinical trial unethical requires specific knowledge about what the trial did wrong. What was the ethical violation? Did it not have proper informed consent? Did it harm participants? Did it violate the Helsinki Declaration or other ethical principles that govern human subjects research?
According to the available reporting, the answer to all of these appears to be no. The trial followed standard procedures. It had informed consent. It compared a new vaccine to something already approved. The ethical violations Makary was pointing to were apparently... not actually there.
But Makary's public suggestion that the trial was unethical carries weight. He's the FDA Commissioner. When he says something on national television, people listen. Companies listen. Investors listen. And when the FDA's top leadership is calling a vaccine trial unethical without specifying concrete violations, it signals that the agency isn't committed to transparent, predictable review. It signals that political appointees might make determinations based on ideology rather than evidence.

The Pattern: Nine Companies and Counting
Moderna isn't alone. Moderna is at least the ninth company to receive an unexpected rejection from Prasad and his team. This isn't a coincidence. This isn't a few isolated disagreements. This is a pattern. And patterns matter because they show intent.
If Prasad had rejected one company's submission and there were solid scientific reasons, you'd evaluate those reasons on their merits. But when a regulator starts rejecting company after company in ways that surprise the agency's own scientists, it suggests something else is going on. It suggests a pattern of using regulatory authority for purposes other than ensuring safety and efficacy.
The industry is noticing. Pharmaceutical companies, biotech firms, and investors are all aware of what's happening. And they're making decisions accordingly. Companies that might have invested in vaccine development are reconsidering. Investors are becoming more cautious. The entire vaccine development ecosystem is contracting in response to regulatory unpredictability.
This has real consequences. We're heading into a future where pandemic threats will probably keep emerging. We need companies working on next-generation vaccines. We need investment in vaccine infrastructure. We need continuous innovation. When the regulatory environment becomes hostile and unpredictable, all of that slows down. Companies pull back. Investment dries up. Years later, when we're facing a new pathogen, we'll wish we had made different decisions about how to treat the companies trying to build countermeasures.

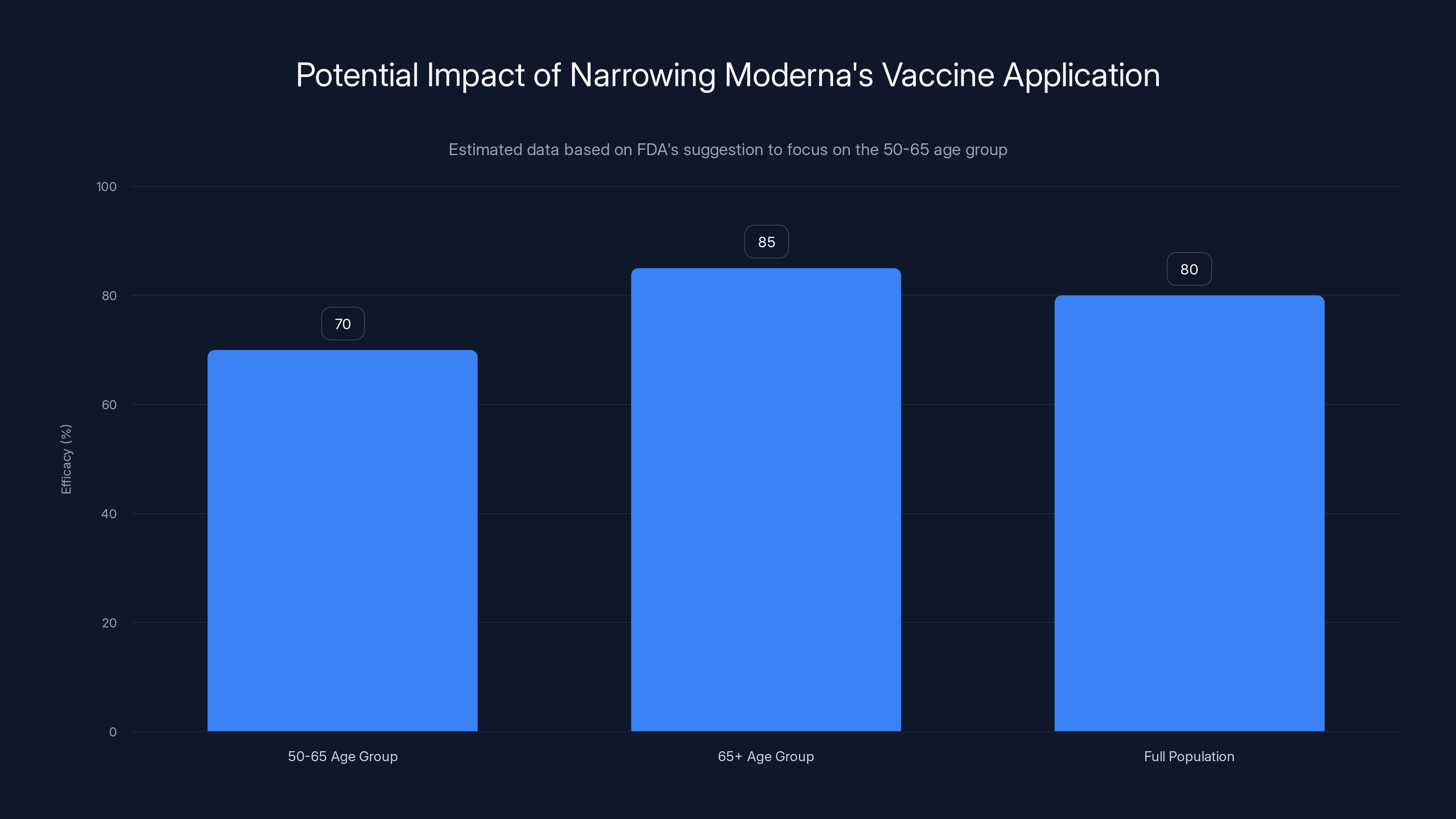
Focusing only on the 50-65 age group may reduce the overall efficacy of Moderna's vaccine application, as older adults (65+) are at higher risk and may benefit more. Estimated data.
The Path Forward: Can Moderna Revive Its Application
So what happens next? Is Moderna's vaccine dead? Not necessarily. According to FDA officials who spoke to Stat News, there might be a path forward. If Moderna shows some "humility"—that's the actual word used—and comes back with a revised submission looking only at the 50-65 age group instead of the full population, the review team might reconsider.
Let that sink in. A company that ran a successful trial, that got the FDA's preliminary approval for its design, that adapted based on feedback, that generated massive amounts of data is now being told that to get a review, it needs to shrink its submission and essentially apologize for following the process.
This is regulatory gaslighting. The FDA was asked what Moderna should do. Moderna did it. And now they're being told they did it wrong and need to have "humility" about it. If you were Moderna's CEO, what would you take away from this interaction? That the FDA's process is fair and predictable? Or that you need to be prepared for moving goalposts and arbitrary decision-making?
It's worth noting that the FDA official's suggestion—focusing only on the 50-65 age group—would actually make the vaccine application weaker. You'd be giving up data on a population that particularly needs better vaccine options. Older adults are at higher risk of serious flu complications. They're the population most likely to benefit from a better vaccine. By suggesting that Moderna drop the 65+ data, the FDA official is essentially asking the company to intentionally weaken its application.

Inside the FDA: Trust Issues and Workplace Concerns
What's happening at the FDA's vaccine division goes beyond vaccine policy. It's becoming a workplace issue. Multiple complaints have been filed against Prasad. These aren't rumors or speculation. These are formal complaints involving allegations of sexual harassment, retaliation against subordinates who disagreed with decisions, and verbally berating staff.
When a leader treats people this way, it affects the organization's ability to function effectively. Scientists become reluctant to speak up. They self-censor. They avoid disagreement. The best people sometimes leave to work elsewhere. What you end up with is an organization where the leader's views go unchallenged not because they're right, but because people are afraid to disagree.
This is particularly problematic in a regulatory context. The FDA is supposed to work because it has processes. Scientists evaluate data. Evidence guides decisions. When a leader creates an environment where people are afraid to speak up, those processes break down. You get decisions made by one person instead of being informed by the collective knowledge of career experts.
The complaints against Prasad suggest that's exactly what's happening. The FDA's vaccine scientists aren't staying silent because they agree with Prasad's decisions. They're raising objections. They're writing memos. They're having meetings. But these dissenting voices aren't changing the outcome. And if the workplace culture is hostile to dissent, fewer people will bother speaking up in the future.

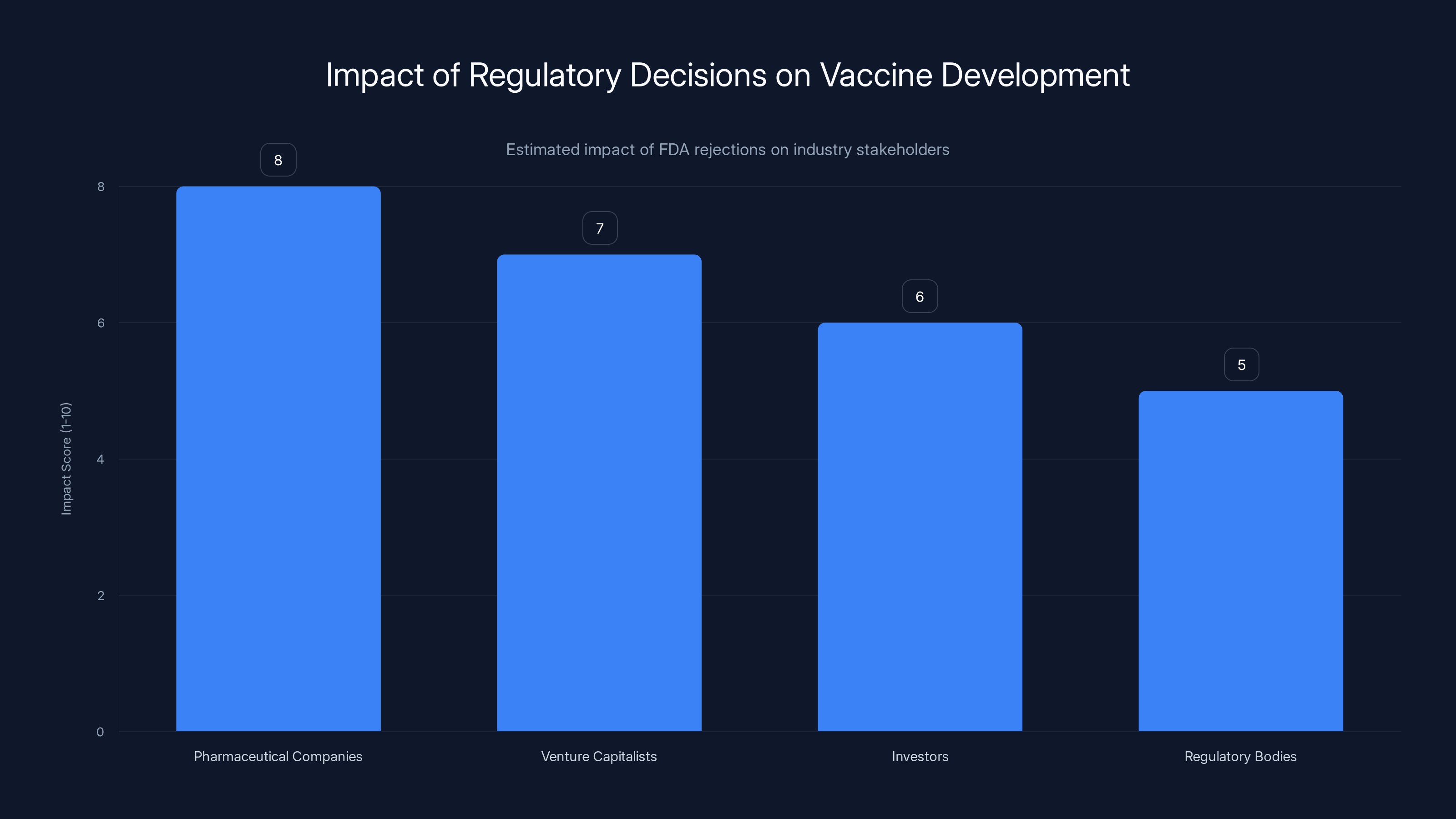
Estimated data shows that FDA rejections based on non-scientific grounds can significantly impact pharmaceutical companies and investors, potentially stifling innovation.
The Broader Implications for Vaccine Development and Public Health
This situation matters for reasons that extend far beyond Moderna. The vaccine industry operates on a business model that requires long-term commitment and substantial investment. Companies make decisions about which vaccines to develop based on expected returns and regulatory risk. When regulatory risk becomes unpredictable, companies start pulling out of vaccines entirely.
We've already seen this happen. There used to be more companies developing vaccines. We've consolidated down to just a handful of major manufacturers. If regulatory risk increases further, we could see additional consolidation. We could see companies decide that vaccines aren't worth developing. We could end up in a situation where we're entirely dependent on a tiny number of manufacturers for our vaccine supply.
This is bad for competition. It's bad for redundancy. It's bad for innovation. And it's particularly bad if we face a genuine pandemic threat and need to rapidly develop new vaccines. In that scenario, we'll wish we'd maintained a robust ecosystem of companies with vaccine development capabilities.
There's also the question of what happens internationally. The United States used to be the center of global vaccine innovation. Companies wanted to develop vaccines here because the FDA's approval meant acceptance worldwide. When the FDA becomes less predictable, less transparent, and less committed to evidence-based decision-making, companies might decide to develop vaccines elsewhere. They might invest in Europe or Asia instead. Over time, that could mean the U.S. ends up more dependent on foreign vaccines, which is the opposite of good public health policy.
The Case for Why Process Matters as Much as Results
You might be thinking: "Okay, but if Prasad is skeptical of vaccines and decides to be tough on vaccine approvals, is that so bad? Shouldn't we want careful evaluation?" This is a fair pushback. Careful evaluation is important. But there's careful evaluation that follows process, and there's arbitrary decision-making dressed up as caution.
The FDA's vaccine review process exists for reasons. It requires transparency. It requires that companies know what's being evaluated and why. It requires that decisions be based on evidence, not on the preferences or ideology of a single appointee. These aren't bureaucratic obstacles. They're features that actually improve decision-making.
When you have a process where scientists can share dissenting views without fear of retaliation, you get better decisions. When regulators have to justify their choices based on evidence rather than personal preference, you get better decisions. When companies know what the rules are and are confident they'll be applied consistently, they make better investment decisions and the ecosystem functions better.
Prasad's approach—rejecting vaccines to "blindside" companies, dismissing concerns about litigation and established practices, managing in ways that discourage dissent—isn't careful evaluation. It's arbitrary exercise of power. And arbitrary exercise of power, even with good intentions, tends to produce worse outcomes than transparent, evidence-based process.
History shows this repeatedly. When regulators become unpredictable, industries respond by becoming more cautious. Investment dries up. Innovation slows. The regulatory agency, trying to be tough, actually ends up weakening the industry it's supposed to oversee.

Comparing Prasad's Approach to Historical FDA Vaccine Review
To understand why this moment is significant, it's worth comparing Prasad's approach to how the FDA has historically handled vaccine reviews. For decades, the FDA's vaccine division developed a reputation for being rigorous but fair. Companies knew what to expect. There were clear guidelines. Scientists at the agency engaged with companies through pre-submission meetings. Feedback was provided prospectively so companies could adjust their trials before enrolling participants.
Was this system perfect? No. But it was predictable. It was transparent. It was based on science and evidence. Companies operating under this system knew that if they did the science right, if they generated good data, the FDA would review it seriously and make a decision based on evidence.
Prasad appears to be overturning this entire approach. Under his leadership, companies can't be confident that following the process will result in review. They can't be sure that preliminary feedback from the FDA means their approach is acceptable. They can't assume that decisions will be consistent or transparent. This is a fundamental shift in how the FDA operates.
The question is whether this shift will produce better vaccine policy or worse vaccine policy. Early evidence suggests worse. Companies are already reconsidering vaccine investments. The industry is already pulling back. Years of trust between the FDA and vaccine developers is already eroding. None of this is likely to improve public health.
What This Means for Future Vaccine Development and Innovation
Let's zoom out and think about what this means for the future. We know that infectious diseases remain a threat. We know that pandemics are possible. We know that developing better vaccines is important. And we know that developing better vaccines requires companies willing to invest, take risks, and pursue innovation.
What regulatory environment encourages that? One where companies trust the regulator. One where processes are transparent. One where decisions are based on evidence. One where companies can reasonably predict what will happen if they do things the right way.
What regulatory environment discourages that? One where companies can't trust the regulator. One where processes are opaque. One where decisions seem arbitrary. One where companies can't predict what will happen no matter what they do.
Prasad's approach is creating the second type of environment. And the consequences will be felt years or decades from now when we're facing a new disease and realizing that we didn't have the vaccine development ecosystem we needed because we'd spent years making it unprofitable and unpredictable to develop vaccines.
This isn't about partisanship. It's not about whether you're generally pro-vaccine or skeptical of vaccines. It's about whether you want a functioning system where evidence guides decisions and processes are transparent. If you do, then what's happening at the FDA is concerning. It suggests that regulatory decisions are being made in ways that don't reflect the traditional commitment to evidence and process.

FAQ
What is Vinay Prasad's background, and why is it relevant to his role as FDA vaccine regulator?
Vinay Prasad is a blood cancer specialist with no formal training or background in vaccine development, immunology, or vaccine regulation. He was appointed as Trump's FDA vaccine regulator in 2025 despite lacking the traditional expertise expected for the position. His background is relevant because vaccine regulation requires specific knowledge of clinical trial design, immunology, epidemiology, and statistical analysis of large-scale trials. When a regulator without this background makes decisions that contradict the recommendations of career scientists, it raises questions about whether decisions are being made based on scientific evidence or other factors.
Why did Prasad reject Moderna's flu vaccine application, and was it based on safety concerns?
Prasad rejected Moderna's flu vaccine application not based on safety or efficacy concerns, but on disagreement with the comparison vaccine Moderna chose to use in their Phase 3 clinical trial. Moderna used a standard-dose flu vaccine as the comparator, which the FDA's own scientists had previously deemed acceptable. This is particularly significant because the FDA had reviewed Moderna's trial design multiple times before the study began and provided preliminary approval. Prasad's rejection thus changed the rules after the trial was already underway and completed with nearly 41,000 participants.
What role did FDA career scientists play in responding to Prasad's decision?
FDA career scientists formally objected to Prasad's rejection. David Kaslow, a senior FDA official, wrote a detailed memo opposing Prasad's decision and explaining why the vaccine review should proceed. Additionally, multiple FDA scientists requested and had an hour-long meeting with Prasad in early January 2025 to present their concerns and lay out their scientific reasoning. Despite these formal objections and documented disagreement from the agency's own experts, Prasad moved forward with his refusal letter, signaling that career scientists' input was being overridden.
Is this the only company Prasad has rejected, or is there a broader pattern?
Moderna is at least the ninth company to receive an unexpected rejection from Prasad and his team. This pattern is significant because it suggests this isn't an isolated decision based on specific concerns about one vaccine, but rather reflects a broader strategy. According to reporting, Prasad told FDA staff he wants to send more refusal letters that "blindside" drug developers, indicating that surprise rejections are part of his intentional approach to regulation. The pattern raises concerns about unpredictable regulatory decision-making within the vaccine division.
What are the broader implications of this decision for vaccine development and the pharmaceutical industry?
Unpredictable regulatory decisions discourage investment and innovation in vaccine development. The pharmaceutical industry makes substantial long-term investments based on expected returns and regulatory risk assessment. When regulatory decisions become unpredictable—when companies can't be sure that following established processes will result in review, or that preliminary FDA approval of trial designs will be honored—companies reconsider their vaccine investments. This could lead to reduced vaccine development, consolidation of manufacturers, and reduced U.S. capacity to respond to future pandemic threats. The industry views Prasad's approach as introducing significant uncertainty into an already complex business model.
Could Moderna's vaccine still be approved, and what would that process look like?
According to FDA officials who spoke to reporters, Moderna might be able to revive its application if it comes back with a revised submission focused only on the 50-65 age group (rather than the full 50+ population) and demonstrates "humility" about not initially following FDA recommendations. This suggests the vaccine itself isn't viewed as unsafe or ineffective, and that a path forward exists. However, this approach would require Moderna to drop data on adults 65 and older, who are actually the population most likely to benefit from improved flu vaccines due to higher risk of serious complications. This outcome would weaken rather than strengthen the application.
What do workplace complaints against Prasad tell us about his management and decision-making?
Multiple formal complaints have been filed against Prasad, including allegations of sexual harassment, retaliation against subordinates who disagree with decisions, and verbally berating staff. These complaints are relevant because they suggest an organizational culture where dissent is discouraged through fear rather than evaluated on merit. When a regulator creates an environment where scientists are afraid to voice disagreement, the decision-making process becomes less reliable. Scientists self-censor. Alternative perspectives aren't heard. The result is that decisions, including regulatory decisions about vaccines, may reflect one person's preferences rather than the collective expertise of the organization's scientific staff.
How does Prasad's approach differ from traditional FDA vaccine review processes?
Historically, the FDA's vaccine division operated with clear, transparent processes. Companies could have pre-submission meetings with FDA scientists to understand what would be required. Trial designs were reviewed prospectively, and feedback was provided before trials began. Decisions were based on evidence and followed consistent standards. Prasad's approach appears to differ fundamentally—using surprise rejections as a tactic, changing standards mid-process (after trials are already complete), and dismissing concerns about deviation from established practices. This represents a significant shift from transparency and predictability toward what appears to be more arbitrary decision-making.
Why is regulatory predictability important for vaccine development and innovation?
Vaccine development requires companies to make long-term commitments and substantial investments years before they see any return. Companies need to know what the regulatory standards are so they can design trials accordingly. When a regulator is unpredictable—changing requirements mid-trial, using surprise rejections, or applying standards inconsistently—companies become less willing to invest. This slows innovation, reduces competition, and ultimately leaves the U.S. more vulnerable to future pandemic threats. A tough but predictable regulator is actually preferable to a lenient but arbitrary one, because companies can plan around consistent standards.
What could happen to the FDA's vaccine division's institutional reputation and staff morale as a result of these decisions?
Prasad's decisions and management style are already affecting institutional reputation and staff morale. Career scientists who spoke out about the Moderna rejection are working in an environment where their recommendations are overruled and their expertise is not valued. This typically leads to some combination of: scientists self-censoring to avoid conflict, the best scientists leaving to work elsewhere, reduced institutional knowledge as experienced people depart, and decreased willingness of future appointees to listen to career staff. Over time, this erodes the FDA's capacity to make good regulatory decisions, even if leadership changes in the future.

The Bottom Line: What Happens Now
The rejection of Moderna's flu vaccine review isn't primarily about Moderna or that specific vaccine. It's a signal to an entire industry. It's a message that regulatory decisions might be made in ways that don't reflect scientific evidence or transparent processes. It's a warning that companies need to factor in unpredictability when deciding whether to invest in vaccine development.
Vinay Prasad has the authority to make these decisions. He's the political appointee leading the FDA's vaccine division. He has every legal right to reject or approve vaccine applications. But having the right to make a decision doesn't mean the decision is good policy. And Prasad's approach—dismissing career scientists' expertise, using surprise rejections as strategy, telling staff he wants to blindside companies, managing through intimidation—suggests decisions are being made in ways that undermine rather than strengthen American vaccine capacity.
This matters because we don't know what threats we'll face ten years from now. We know that infectious diseases continue to emerge. We know that pandemics are possible. We know that when those threats arrive, we'll need companies with vaccine development capability. By making vaccine development unprofitable and unpredictable today, we're making ourselves less prepared for whatever comes next.
The question now is whether the FDA's institutional leadership, whether Congress, and whether the broader stakeholder community will address what's happening. Will the agency's processes be corrected? Will standards be clarified? Will oversight be implemented to ensure regulatory decisions reflect evidence rather than personal preference? Or will this become the new normal, with companies continuing to pull back from vaccine development and the U.S. gradually losing its position as a leader in vaccine innovation?
Those are the questions that really matter from this situation. Because unlike the question of whether one vaccine gets approved, those answers will shape whether we're prepared when the next pandemic arrives.

Key Takeaways
- Vinay Prasad, Trump's FDA vaccine regulator with no vaccine expertise, rejected Moderna's mRNA flu vaccine review despite FDA career scientists recommending approval
- The rejection wasn't based on safety concerns but on disagreement with Moderna's choice of comparison vaccine in a 41,000-person trial that FDA had previously approved
- FDA career scientist David Kaslow formally objected to Prasad's decision in writing, and multiple scientists had an hour-long meeting with Prasad presenting their concerns
- Prasad reportedly told FDA staff he wants to issue more surprise rejections to 'blindside' drug developers, breaking with FDA's established transparent processes
- Moderna is at least the ninth company to receive unexpected rejections from Prasad's office, raising industry concerns about regulatory unpredictability and future vaccine development investment
Related Articles
- Trump's 'Buy American' EV Charging Rule: A De Facto NEVI Moratorium [2025]
- Ivermectin as Cancer Treatment: Why Federal Research Funding Raises Red Flags [2025]
- Live Nation's Monopoly Trial: Inside the DOJ's Internal Battle [2025]
- RFK Jr.'s Autism Panel & Vaccine Conspiracy Theories [2025]
- Public Health Workers Resigning Over ICE & Guantánamo Assignments [2025]
- Raw Milk During Pregnancy: Health Risks, Listeria Dangers & Why Pasteurization Matters [2025]
![FDA Vaccine Official Overruled Scientists on Moderna Flu Shot [2025]](https://tryrunable.com/blog/fda-vaccine-official-overruled-scientists-on-moderna-flu-sho/image-1-1770937804559.jpg)



