Introduction: A Shocking Reversal That Exposed the Politics of Vaccines
In February 2025, something unprecedented happened at the Food and Drug Administration. A political appointee with the Trump administration simply refused to review Moderna's mRNA flu vaccine—not because the science was flawed, but because of a disagreement over which comparison vaccine the company used in its clinical trial. Then, just days later, the FDA reversed that decision entirely.
This wasn't a normal regulatory hiccup. It was a window into how political pressure is reshaping vaccine development in America, and the stakes couldn't be higher. Moderna had invested years and millions into developing an mRNA-based flu vaccine. The company had conducted a rigorous Phase 3 trial involving nearly 41,000 adults aged 50 and older. FDA career scientists had reviewed the trial design and signed off on it. Everything was in order. Then Vinay Prasad, the Trump administration's top vaccine regulator, said no.
The decision shocked agency insiders, alarmed public health experts, and sent a troubling signal to the entire vaccine industry. But what made this moment even more significant was what came next. After Moderna made strategic changes to its application—proposing to split it into two pathways for different age groups—the FDA suddenly agreed to move forward. The reversal happened so quickly it felt less like scientific reconsideration and more like political capitulation.
This story matters far beyond Moderna's bottom line. It reveals deep fractures within the FDA between political appointees and career scientists. It shows how vaccine companies are operating under unprecedented uncertainty. And it raises urgent questions about whether America's vaccine innovation pipeline can survive the current political climate. The implications stretch from boardrooms in Cambridge to public health offices across the country, affecting everything from next winter's flu season to the future of mRNA technology itself.
In this comprehensive analysis, we'll examine what actually happened, why it happened, what it means for vaccine development, and what happens next. The vaccine debate in America has always been contentious, but 2025 marks a turning point. Understanding this moment is essential to understanding the future of American medicine.
TL; DR
- Political Pressure Overruled Science: Trump administration vaccine chief Vinay Prasad rejected Moderna's mRNA flu vaccine application despite FDA scientists supporting review, citing issues with the trial's control vaccine
- The Reversal Came Fast: After Moderna proposed splitting the application into separate pathways for different age groups, the FDA reversed course within days, agreeing to advance the review
- Timeline: Decision expected by August 5, 2026, with vaccine potentially available to seniors by late 2026
- Broader Context: The reversal reflects deeper tensions between political appointees and career FDA scientists, plus growing anti-mRNA sentiment within the Trump administration
- Industry Impact: Vaccine companies are abandoning research and cutting jobs as uncertainty spreads; Moderna has already lost $700 million in federal contracts
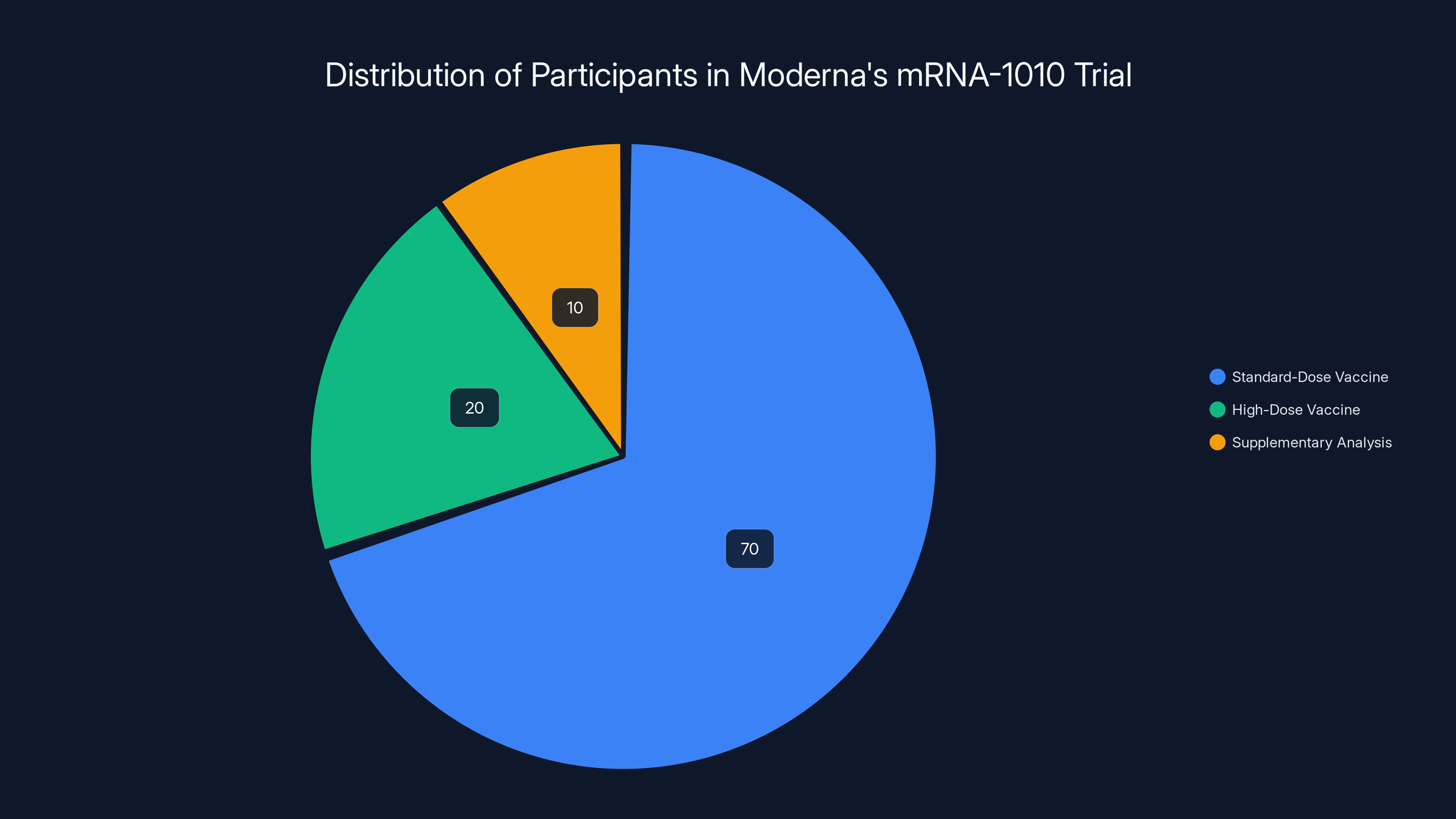
Estimated data shows the majority of participants received the standard-dose vaccine, with a smaller portion involved in supplementary analysis using a high-dose vaccine.
What Actually Happened: The Rejection That Shocked the FDA
On February 3, 2025, Vinay Prasad, serving as the Trump administration's chief vaccine regulator, sent a letter to Moderna with a single message: the FDA would not review the company's mRNA flu vaccine application. The decision was shocking because it wasn't based on the vaccine itself—it was based on the flu vaccine Moderna used for comparison in its clinical trial.
Moderna's vaccine candidate, designated mRNA-1010, had been tested against licensed standard-dose influenza vaccines, including Fluarix manufactured by GlaxoSmithKline. This was the choice Prasad objected to. He argued that using a standard-dose vaccine "does not reflect the best-available standard of care" and therefore the entire trial wasn't "adequate and well-controlled."
Here's the thing that makes this particularly confusing: the FDA had already approved this trial design. Before Moderna conducted the Phase 3 trial involving nearly 41,000 participants aged 50 and older, FDA scientists had reviewed the proposed methodology. During those discussions, the agency had suggested that Moderna consider using a high-dose flu vaccine for participants aged 65 and older—people who benefit most from stronger immune responses. Moderna acknowledged the suggestion, but the FDA ultimately signed off on the company's plan to use the uniform standard dose across all participants. It was acceptable. It was allowed. It was agreed to.
But now, with the trial complete and data in hand, Prasad was rejecting the entire thing because of that same design choice the agency had previously accepted.
Moderna, for its part, wasn't unprepared. The company had anticipated this possibility and had already conducted additional analyses. They agreed to compare their vaccine's performance against a high-dose alternative in some older participants and provided the FDA with supplementary data addressing the control vaccine question. In other words, they were trying to meet the objections head-on.
Inside the FDA, the response was chaos. A team of career scientists—the people who actually review vaccine applications and understand the immunological data—prepared their analysis. They held an hour-long meeting with Prasad to explain why the review should proceed. David Kaslow, a top career official responsible for vaccine review, even wrote a memo detailing the scientific rationale for moving forward with the application review.
Prasad rejected all of it. He rejected the scientists' recommendation. He rejected the memo. He rejected the compromise data Moderna offered. The answer remained no.
What happened next revealed something important about how power actually works in government agencies. Word spread quickly about the shocking decision. Moderna issued a sharply worded press release that essentially said: "We got rejected, and we don't think this is fair." Reporting by journalists—digging into the decision's actual rationale—uncovered that a political appointee had overruled career scientists. That's not how the FDA normally operates. The agency takes a deliberate, consensus-driven approach. Surprising rejections are rare. Rejecting applications outright, without review, is practically unheard of.
The decision started looking less like a scientific concern and more like something else entirely.
Who Is Vinay Prasad and Why Does His Position Matter?
Vinay Prasad isn't just another bureaucrat in the federal health apparatus. He serves as the chief medical officer and head of the vaccine division within the Trump administration's health infrastructure. That position gives him enormous power over which vaccines get reviewed, how quickly they're evaluated, and ultimately whether they reach patients.
Prasad's background matters here. He's worked in various regulatory and clinical roles, but what's most relevant is his apparent skepticism of newer vaccine technologies, particularly mRNA platforms. In the context of the Trump administration's broader vaccine agenda—which has shifted dramatically from the previous administration's approach—Prasad represents something of an ideological realignment at the agency.
The memo from David Kaslow, the career official who recommended reviewing the Moderna application, carried significant weight because Kaslow has deep expertise in vaccine immunology and has served in regulatory capacities for decades. When someone of Kaslow's stature writes a memo saying a vaccine application should move forward, that carries institutional credibility. That credibility is precisely what Prasad overrode.
This created an unprecedented situation: a political appointee in the Trump administration making unilateral decisions that contradicted the recommendations of career scientists with specialized expertise. That's not a novel problem in government generally, but it's unusual in vaccine regulation, where scientific consensus has historically been determinative.
The larger context is crucial. The Trump administration came into office with Robert F. Kennedy Jr. as Secretary of Health and Human Services. Kennedy has built his political brand on skepticism of vaccines, particularly newer mRNA technology. He's worked to install allies throughout federal health agencies who share that skepticism. Prasad appears to be one of those allies. His rejection of Moderna's vaccine application aligns perfectly with Kennedy's broader anti-vaccine agenda, even if the stated rationale was about trial methodology.
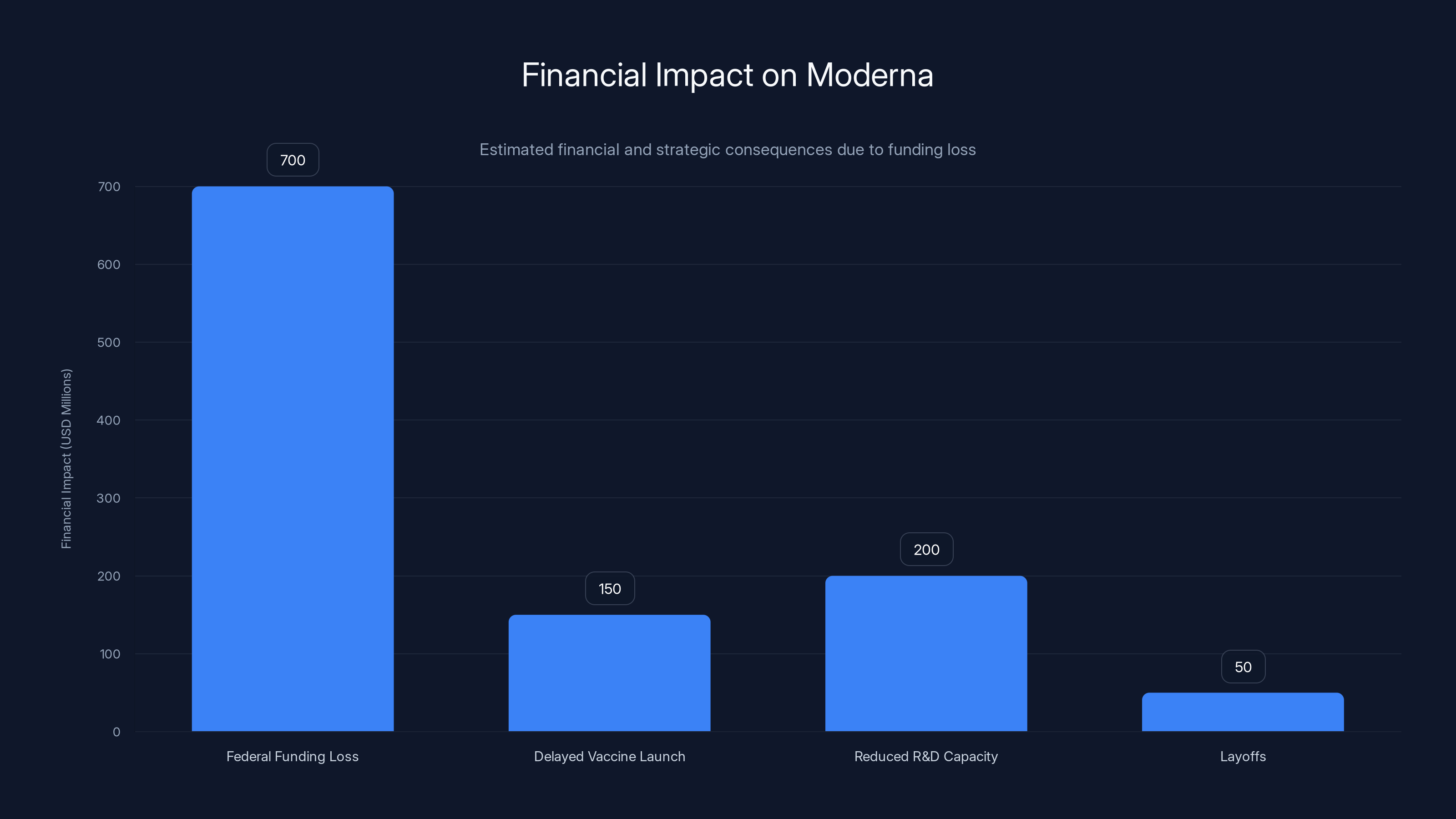
Moderna faces a significant financial impact with an estimated $700 million federal funding loss, affecting R&D capacity and resulting in layoffs. Estimated data.
The Reversal: How a Phone Call Changed Everything
Just days after the rejection shook through the industry, Moderna held what's called a "Type A meeting" with the FDA. These formal regulatory meetings are structured conversations between companies and the agency designed to resolve disagreements and find paths forward. They're typically productive precisely because they bring together different perspectives in a structured format.
During this meeting, Moderna proposed a strategic pivot. Instead of seeking a single approval for its mRNA flu vaccine across all age groups, the company would split the application. They would seek full approval for use in people aged 50 to 64—a group where the existing trial data was robust and straightforward. For people aged 65 and older—the group Prasad had fixated on—Moderna would use an accelerated approval pathway.
Accelerated approval is a regulatory mechanism designed for serious diseases or public health needs. It allows vaccines to reach patients faster while the company commits to conducting additional studies after approval to confirm effectiveness. In the case of seniors and flu vaccination, this made sense. The elderly face the highest risk from influenza complications, so an accelerated pathway that got a new vaccine option to them faster had public health logic.
When Moderna proposed this split approach, something shifted. The FDA agreed to review the application. Andrew Nixon, a spokesperson for the Department of Health and Human Services, confirmed the reversal in a terse statement: "Discussions with the company led to a revised regulatory approach and an amended application, which FDA accepted."
That's an interesting phrasing. "Discussions led to a revised regulatory approach." It's not saying the science changed. It's not saying the FDA discovered new data. It's saying that after talking about it, both sides found a way to move forward. In regulatory terms, that often means: "We found a way for both sides to save face and keep moving."
The FDA set an expected decision date of August 5, 2026—about six months of review time. If approved, Moderna expected to make the vaccine available to seniors by later in 2026. That's a compressed timeline compared to typical vaccine approvals, but it reflected the urgency both Moderna and (eventually) the FDA felt about getting a new flu vaccine option to the market.
What's striking about the reversal is how quickly it happened. Prasad didn't slowly come around to the scientific argument. The argument about trial methodology didn't gradually convince him that review was appropriate. Instead, a different regulatory pathway suddenly made the same vaccine application acceptable. That suggests the objection to the trial design may not have been the actual barrier—or at least, not the primary one.
The Context: Why This Rejection Happened Now
To understand why Prasad rejected the application and why the reversal mattered so much, you need to understand the broader political context around vaccines and mRNA technology in the Trump administration.
Robert F. Kennedy Jr. entered his role as Secretary of Health and Human Services with an explicit anti-vaccine agenda. He's built a political career on spreading doubt about vaccine safety, particularly around newer technologies like mRNA. Kennedy's views diverge sharply from mainstream public health science. The scientific consensus overwhelmingly supports mRNA vaccines as safe and effective. Kennedy's position is that mRNA vaccines are linked to rising rates of acute and chronic illness—a claim not supported by epidemiological evidence.
But Kennedy doesn't just hold personal opinions. He has the power to implement policy. He's installing allies in key positions throughout federal health agencies. He's funding institutions that promote vaccine skepticism. His MAHA Institute—MAHA stands for Make America Healthy Again—is hosting events that claim there's a "massive epidemic of vaccine injury" linked to mRNA vaccines. None of these claims have credible scientific support, but that's not the point. The point is that Kennedy is using institutional and political power to marginalize mRNA vaccine development.
Moderna, as the leading mRNA vaccine company, is caught directly in the crosshairs. The company has lost more than $700 million in federal contracts to develop pandemic vaccines. That's not a rounding error—that's real money that was expected to fund research and development. Those contracts were cancelled or defunded as part of the broader shift in federal vaccine policy.
For companies considering new vaccine development, the message is clear: the political winds have shifted. An mRNA vaccine that might have sailed through review under the previous administration now faces ideological resistance. Prasad's rejection of Moderna's application wasn't scientifically justified—career scientists disagreed with him—but it was politically effective. It signaled that mRNA vaccines face obstacles in the current regulatory environment.
That signal is having real consequences across the industry.
The Scientific Question: Was Prasad's Objection Valid?
Let's examine the actual scientific merit of Prasad's objection, because it matters whether there was any legitimate concern buried under the political context.
Prasad argued that Moderna should have used a high-dose flu vaccine as the comparison in its clinical trial, at least for participants aged 65 and older. High-dose vaccines are approved for senior populations because research shows they generate stronger immune responses in older adults, whose immune systems don't respond as robustly to standard-dose vaccines. The idea makes sense: if you're testing a new vaccine for seniors, shouldn't you compare it to the best option available for that population?
But here's where the regulatory logic becomes important. The FDA had already approved the trial design using a standard-dose comparison. Companies, the FDA, and outside experts had discussed the approach and decided it was appropriate. Standard-dose vaccines are still effective and are widely used. Comparing a new vaccine to a standard option that's already in use provides valuable real-world data. The FDA agreed to this methodology before the trial began.
Moreover, Moderna didn't ignore the high-dose question. The company had data on how their vaccine performed against high-dose alternatives in some trial participants. They offered to provide additional analysis comparing their vaccine to high-dose options. They were willing to incorporate that data into their submission. In other words, they addressed the underlying concern—that you should understand how their vaccine stacks up against high-dose competitors—without requiring the entire trial to be redesigned.
FDA career scientists, reviewing the data and the situation, concluded that the trial design was acceptable and that Moderna's additional analysis addressing the high-dose question was sufficient. That's a scientifically defensible position. The trial compared the new vaccine to an approved standard. The company provided supplementary data about high-dose comparisons. The methodology was reasonable.
Was it perfect? Nothing in science ever is. Could the trial have been designed differently? Sure. Would a comparison to high-dose vaccines have provided valuable additional information? Absolutely. But "could have been better designed" is not the same as "inadequate and not well-controlled."
Presumably, Prasad knew this. Or at least, the career scientists who briefed him certainly made this argument. That he rejected it anyway suggests his objection may not have been purely scientific. The subsequent reversal—triggered not by new data but by a change in regulatory strategy—further suggests that the trial design concern may have been a pretext rather than the actual barrier.
This matters because it shapes how we interpret what happened. If Prasad had genuinely believed the trial was scientifically deficient, he would presumably maintain that position regardless of the regulatory pathway. Instead, the same vaccine, based on the same trial, became acceptable once Moderna proposed a different approach to seeking approval. That pattern suggests politics rather than science drove the initial decision.
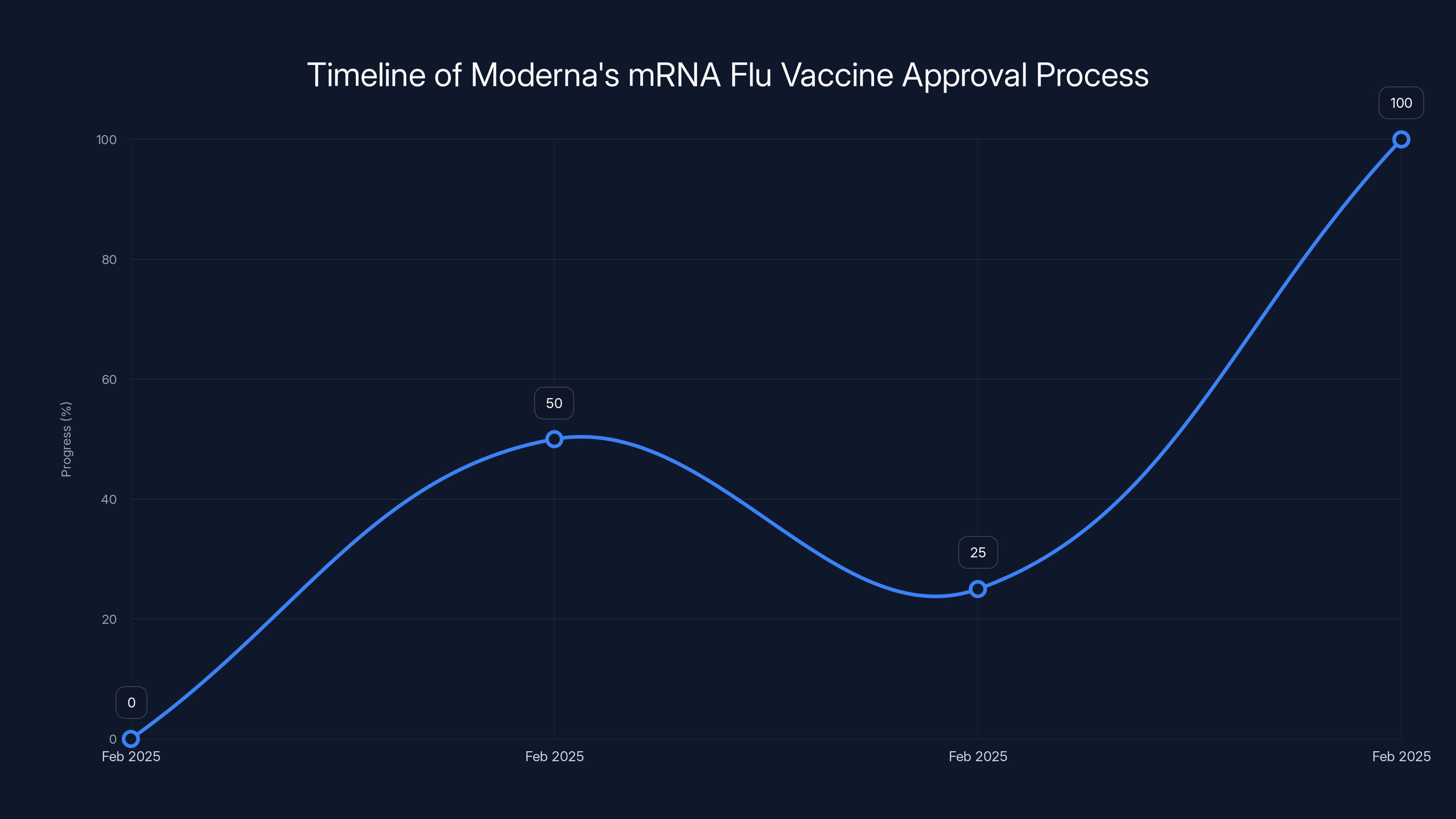
Estimated data: The timeline illustrates the abrupt changes in Moderna's mRNA flu vaccine approval process, highlighting the political influence on regulatory decisions.
Impact on Moderna: Financial and Strategic Consequences
For Moderna, the rejection, even with the subsequent reversal, created real damage. The company had been planning to launch its mRNA flu vaccine as a significant commercial product, offering seniors a new option and potentially capturing meaningful market share in the flu vaccine space. Uncertainty delays that timeline and creates complications.
But the financial impact goes far beyond one vaccine. Moderna had counted on federal contracts to fund pandemic vaccine development—work that positions the company as a critical infrastructure asset in public health. Those contracts provided both revenue and strategic flexibility. Losing more than $700 million in federal funding represents a major setback. That money was supposed to fund research into next-generation vaccines and pandemic preparedness. Without it, Moderna has less capacity to fund that work.
The company's president, Stephen Hoge, articulated the concern clearly when speaking to the New York Times. "There will be less invention, investment, and innovation in vaccines generally, across all the companies," he said. That's not hyperbole. When a major vaccine manufacturer loses hundreds of millions in federal funding and faces regulatory hostility toward its core technology platform, it scales back ambitious research. It postpones new projects. It lays off scientists who were working on next-generation vaccines.
Moderna's experience signals to the entire industry what happens when you pursue mRNA vaccine development under a Trump administration skeptical of the technology. Smaller companies that might have considered entering the mRNA vaccine space now face a sobering calculation. The scientific case for mRNA vaccines is strong, but the political winds are unfavorable. Why invest years and tens of millions in a new mRNA vaccine program if the regulatory environment is hostile and federal funding is evaporating?
This is how policy shapes innovation. Not through direct prohibition, but through creating an environment where certain types of research become economically and politically unviable. Companies that might have invested in mRNA flu vaccines, mRNA RSV vaccines, mRNA cancer vaccines, or other mRNA therapeutics now face pressure to reallocate resources to platforms that don't carry the same political risk.
For Moderna specifically, the reversal was necessary to prevent the company from abandoning the mRNA flu vaccine entirely. But it came with a cost: the company had to accept an accelerated approval pathway for seniors, which means additional work and uncertainty beyond the initial approval. That's not ideal from a business perspective, but it's better than no approval at all.
Ripple Effects: How This Decision Shaped the Entire Vaccine Industry
The Moderna rejection and reversal didn't happen in isolation. It sent signals throughout the vaccine industry about how the regulatory environment has shifted. Those signals are creating real consequences.
Vaccine companies and biotech firms working on vaccine development are actively making decisions based on the apparent hostility toward mRNA technology. Researchers who built careers in mRNA vaccine development are exploring other opportunities. Companies that had allocated resources to vaccine programs are redirecting that capital. Job cuts are happening. Research programs are being paused.
This represents a significant shift from recent history. Between 2020 and 2024, mRNA vaccine technology was ascendant. The success of COVID-19 mRNA vaccines from Moderna and Pfizer created enormous enthusiasm for applying the platform to other diseases. Companies were investing in mRNA vaccines for influenza, RSV, malaria, tuberculosis, and cancer. Government agencies were funding research. The field felt momentum.
That momentum is stopping. The Moderna situation crystallized something many in the industry had been watching nervously: the Trump administration's stated skepticism of mRNA vaccines wasn't just political posturing. It was translating into actual regulatory and funding decisions. That changes behavior.
When the chief vaccine regulator rejects an application by the leading mRNA vaccine company, and when that rejection appears to be ideologically motivated rather than scientifically grounded, it sends a message. The message is: mRNA vaccines are not going to get favorable treatment in this regulatory environment. Anyone investing in mRNA vaccine development should expect obstacles.
The consequences will take years to fully materialize. A researcher deciding today not to pursue mRNA vaccine research means fewer papers published in five years. A biotech company deciding to pause an mRNA vaccine program means that program won't reach clinical trials for a decade, if ever. A venture capital firm deciding to reduce vaccine investments means fewer startups pursuing ambitious vaccine projects.
Vaccine development is a long-term endeavor. The decisions being made now, in response to the Moderna situation and broader anti-vaccine signals from the Trump administration, will shape which vaccines get developed, which technologies get pursued, and which diseases we can effectively vaccinate against a decade from now.
The Vaccine Safety Question: What Does the Evidence Actually Show?
Underlying the entire Moderna situation is a fundamental disagreement about vaccine safety. Kennedy and the Trump administration's anti-vaccine allies argue that mRNA vaccines are linked to rising rates of acute and chronic illness. That claim is central to their skepticism of the technology. So it's worth examining what the evidence actually shows.
Moderna and Pfizer's COVID-19 vaccines have been administered billions of times globally. That's a dataset of unprecedented scale. Researchers have conducted extensive post-authorization surveillance, tracking adverse events and safety signals. The evidence is clear: the vaccines are safe. Serious adverse events are extraordinarily rare. The most common side effects—arm soreness, fatigue, mild fever—are mild and temporary.
Could mRNA vaccines be linked to some rare condition that hasn't been detected yet? Theoretically, any medication could have rare side effects that take time to identify. That's why safety monitoring is continuous. But the claim that mRNA vaccines are causing "rising rates of acute and chronic illness" isn't supported by epidemiological data. Disease incidence rates haven't mysteriously jumped in vaccinated populations compared to unvaccinated ones.
There are legitimate scientific discussions about which vaccines work best for which populations, about optimal dosing schedules, about comparative effectiveness. These are the kinds of conversations career scientists have all the time. The Moderna trial design discussion is an example of a legitimate scientific question.
But that's very different from the broader anti-vaccine narrative. When Kennedy and allies claim that mRNA vaccines are driving epidemics of chronic disease, they're not making a nuanced scientific argument. They're making a claim that contradicts available evidence.
What makes this relevant to the Moderna situation is that Prasad's rejection appears to be part of a broader effort to marginalize mRNA vaccines based on ideological rather than scientific grounds. The trial design objection may have been a legitimate question, but the swift reversal when Moderna proposed a different pathway suggests it wasn't the actual driver of the decision.
The implications are concerning. If regulatory decisions are being driven by anti-vaccine ideology rather than scientific assessment, that threatens the integrity of the approval process. Companies pursuing vaccines won't know what standards they actually need to meet because the standards are shifting based on political winds rather than scientific criteria.
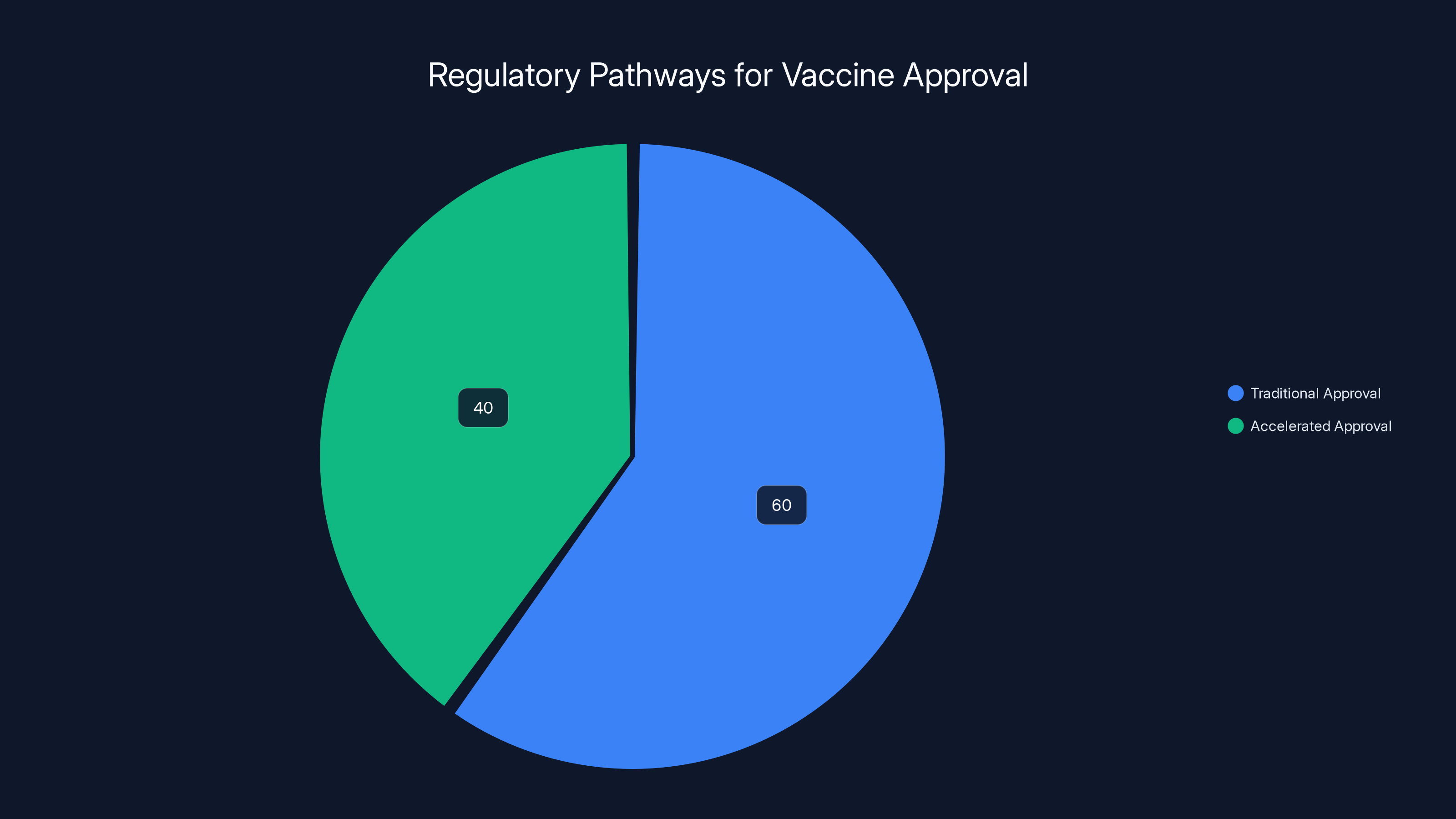
Accelerated approval allows vaccines to reach patients 40% faster than traditional pathways, which is crucial for urgent public health needs. Estimated data.
How Vaccine Trials Work: Understanding the Methodology
To fully appreciate what happened with the Moderna trial design, it's helpful to understand how vaccine trials work and why the choice of control vaccine matters.
A typical vaccine trial follows a rigorous structure. You recruit thousands of participants—in Moderna's case, nearly 41,000 people aged 50 and older. You randomly divide them into groups. Some get the new vaccine. Others get either a placebo or an existing vaccine (the "control"). You follow everyone over time and track who gets sick, what side effects occur, and how immune responses develop.
The control matters enormously. If you compare your new vaccine to a placebo, you can see if your vaccine does better than nothing. But that's a pretty low bar—most vaccines beat nothing. If you compare your new vaccine to an existing approved vaccine, you get information about relative performance. That's much more useful.
For flu vaccines specifically, there's a question about which approved vaccine to use as the comparison. Standard-dose vaccines are widely used and effective across age groups. High-dose vaccines are approved specifically for seniors because they provide stronger immune responses in older people with waning immunity. There are reasonable arguments for using either as a control.
Moderna chose standard-dose. That choice means the trial answers the question: "How does our new vaccine compare to what people are already getting?" That's commercially and practically relevant information. It's also the choice the FDA had previously approved.
But Prasad wanted high-dose—at least for seniors. His argument was essentially: "For older people, you should compare against the best-available option for that population." That's not a crazy objection. It's a legitimate scientific question.
The issue is that Prasad wanted to reject the entire application because of that question, rather than asking Moderna to provide additional analysis. Moderna was willing to do the analysis. The company provided data comparing their vaccine to high-dose alternatives. Career scientists reviewed that data and deemed it sufficient. The question was answerable without redesigning the entire trial.
So Prasad faced a choice: reject the application outright, or allow it to move forward while requesting additional data. He chose rejection. When Moderna proposed a different regulatory pathway, rejection became politically untenable, so he allowed it to proceed.
That pattern—reject, then reverse when faced with reframing—suggests something other than rigorous scientific assessment was driving the decision.
Federal Funding: The Economic Pressure on Vaccine Development
Why did Moderna lose $700 million in federal contracts? The answer involves pandemic preparedness infrastructure that the Trump administration has de-prioritized.
During the COVID-19 pandemic, the federal government invested heavily in vaccine development and manufacturing capacity. Even as the emergency phase ended, there remained a national interest in maintaining pandemic preparedness—keeping vaccine manufacturers ready to rapidly produce new vaccines if a novel pathogen emerged. That seemed like a reasonable insurance policy.
The Trump administration apparently disagrees. It's reducing funding for pandemic vaccine development and manufacturing infrastructure. Those are the contracts Moderna lost. The company had been part of the federal government's pandemic preparedness network, funded to maintain the ability to rapidly develop new vaccines for emerging pathogens.
That's not trivial. Pandemic preparedness is expensive. It requires maintaining manufacturing capacity, funding research into next-generation vaccine platforms, and keeping scientific teams ready to mobilize quickly. If the federal government isn't funding that work, companies have to decide whether it's worth the investment on their own.
Moderna, like other vaccine companies, has concluded it's not. Without federal funding, the company can't justify the cost of maintaining pandemic preparedness capacity. That means slower vaccine development if a new pathogen emerges. It means less innovation in vaccine technology because the funding that drives research isn't there.
The $700 million figure represents the economic cost of de-prioritizing pandemic preparedness. It's also a signal to other vaccine companies: don't expect federal funding for pandemic vaccine development under this administration. Plan accordingly.
That has implications for the entire vaccine industry. Smaller companies, which rely more heavily on federal support, face particularly difficult choices. Do you pursue vaccine development knowing that federal funding has evaporated? Do you maintain expensive research programs hoping the political winds change? Or do you exit the vaccine space entirely?
Many companies are choosing the latter. That's how policy shapes innovation—not through prohibition, but through removing the economic incentives that make certain kinds of work viable.

The Kennedy Effect: Anti-Vaccine Influence Throughout Federal Health Agencies
Robert F. Kennedy Jr.'s influence on federal vaccine policy extends far beyond his position as Secretary of Health and Human Services. He's actively reshaping the entire apparatus through personnel decisions and institutional changes.
Kennedy has installed allies in key positions throughout federal health agencies. These aren't typically people with deep expertise in vaccine immunology or infectious disease. They're people who share Kennedy's skepticism of vaccines and, particularly, mRNA technology. As those people move into positions of authority—over vaccine review, over clinical guidelines, over research funding—they shape decisions.
Vinay Prasad's decision to reject the Moderna application aligns perfectly with Kennedy's agenda. Whether that decision was directly coordinated is unclear, but it didn't happen in a vacuum. Prasad works in an environment where mRNA vaccines are viewed skeptically. His decision to reject the application, using a debatable scientific objection as cover, makes sense in that context.
Kennedy is also funding institutions that promote vaccine skepticism. His MAHA Institute hosts events and funds research that promotes anti-vaccine narratives. The claim that mRNA vaccines are causing epidemics of chronic disease—a claim without strong scientific support—gets amplified through these institutions. Over time, repetition creates an impression of legitimacy even when the underlying evidence is weak.
What's particularly concerning is that Kennedy has influence over federal agencies responsible for vaccine safety and vaccine recommendations. The CDC, for example, issues recommendations about which vaccines people should receive. The FDA approves which vaccines can be administered. When leadership at these agencies includes people skeptical of vaccines, that shapes what gets recommended and approved.
The Moderna situation illustrates this dynamic perfectly. Prasad rejected an mRNA vaccine application using questionable logic. Had he worked in an environment where his decision was constrained by independent review and scientific consensus, it might not have happened. But in an environment where mRNA vaccines are viewed skeptically at the top levels of federal health agencies, his decision fit the broader pattern.
That pattern—installing skeptics in positions of authority, funding institutions that promote vaccine doubt, using administrative decisions to slow vaccine approval—doesn't require explicit coordination. It's simply the natural consequence of having anti-vaccine leadership throughout federal health agencies.
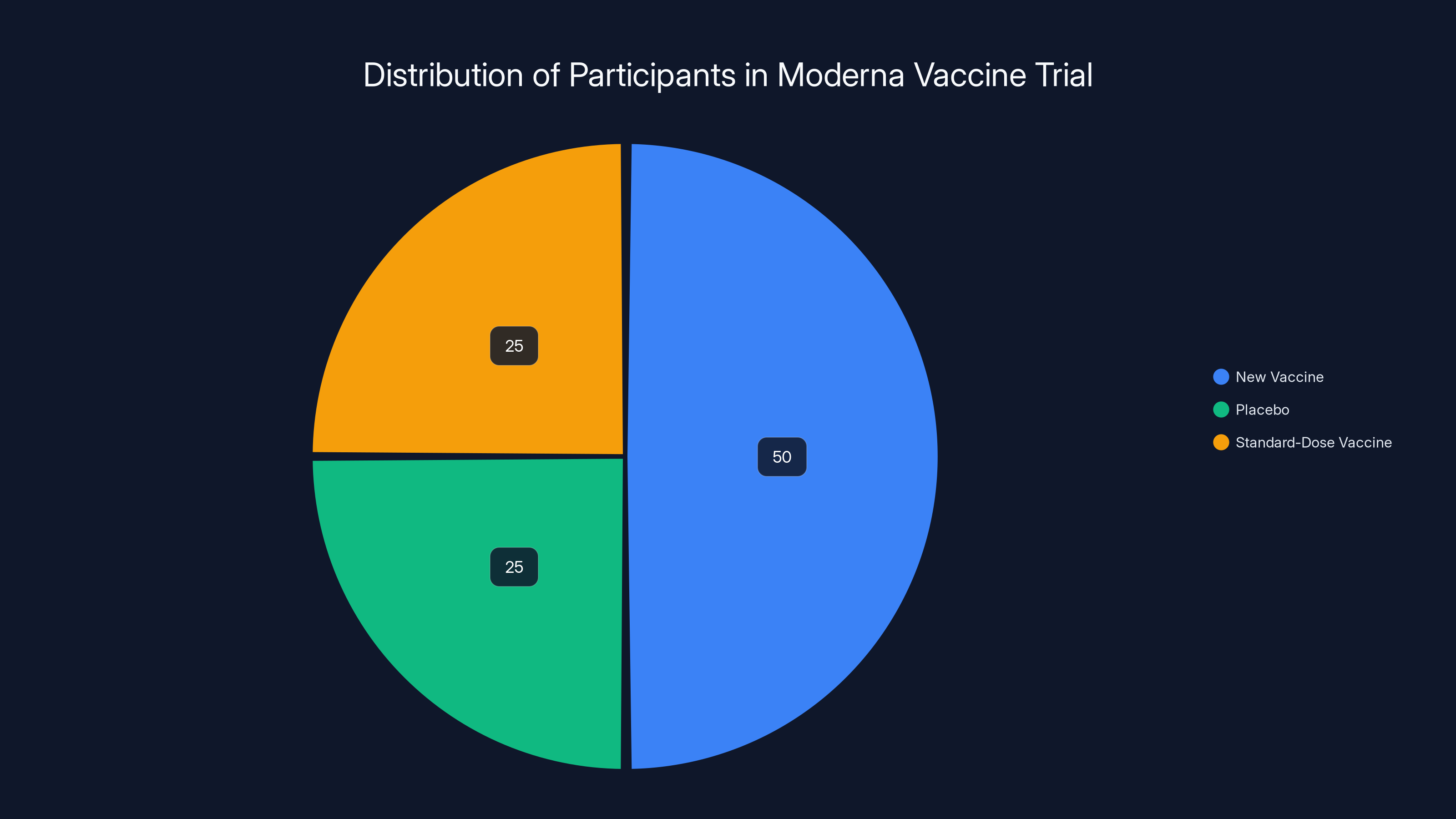
In the Moderna trial, participants were divided into groups receiving the new vaccine, a placebo, or a standard-dose vaccine. Estimated data shows an equal split between placebo and standard-dose groups.
The Reversal: Strategic Maneuvering or Genuine Reconsideration?
When Moderna proposed splitting the application into two pathways—full approval for ages 50-64 and accelerated approval for ages 65+—why did that resolve the stalemate?
One explanation is purely political. The initial rejection had generated negative publicity. Career scientists had made their objections to Prasad's decision public (through reporting). Moderna had issued a critical press release. The optics were bad for the administration. By allowing the application to proceed through a different pathway, Prasad could claim to have resolved the issue without losing face.
That explanation requires assuming that Prasad's initial concern about the trial design wasn't deeply held. It was a position he was willing to abandon if circumstances changed. The circumstances that changed were: a different regulatory pathway was proposed. But the underlying scientific question—whether the trial adequately compared the vaccine to appropriate controls—didn't change. The same vaccine, based on the same trial, suddenly became acceptable.
Another explanation is that the accelerated approval pathway addresses legitimate regulatory concerns. Accelerated approval for seniors, with a commitment to additional studies post-approval, might provide the additional certainty that Prasad wanted. The original single-pathway approach would have given full approval based on the Phase 3 trial. The split approach allows full approval for one group and accelerated approval for another, with additional data collection required before full approval for seniors.
That explanation suggests Prasad had a coherent concern—some seniors might not be adequately protected by the Phase 3 data—and the split pathway addressed it. From that perspective, the reversal represents Prasad getting what he wanted. The company adapts its approach to address his concerns. Everyone moves forward.
The truth is probably somewhere between these explanations. Prasad likely had genuine concerns about the trial design—the accelerated approval pathway does address questions about senior populations specifically. But the intensity of his initial objection—outright rejection rather than requesting additional data—suggests the concern wasn't purely scientific. The reversal came remarkably quickly once a different regulatory framework was available.
What matters is that the decision-making process wasn't transparent. Career scientists made arguments. Those arguments were overruled. The decision was reversed without clear explanation of what changed scientifically. That opacity is corrosive to trust in the regulatory process. Companies and the public can't have confidence in standards that shift based on political winds and can be resolved through regulatory restructuring rather than scientific debate.

Timeline: How This Will Unfold
The FDA has approximately six months to review Moderna's amended mRNA flu vaccine application, with a decision expected by August 5, 2026. That's a compressed timeline compared to typical vaccine reviews, which can take a year or longer. But it's not unprecedented, especially for vaccines addressing serious public health needs.
During that review period, FDA scientists will examine the clinical trial data in detail. They'll scrutinize safety outcomes, efficacy signals, and manufacturing quality. They'll evaluate whether the vaccine candidate meets regulatory standards for approval. They'll probably issue questions and requests for additional information. Moderna will respond. The process will continue iteratively until the FDA is satisfied.
If approved, Moderna expects to have the vaccine available to seniors by later in 2026. That assumes manufacturing can ramp up quickly, which is generally feasible for vaccines that companies have been developing. It also assumes no unforeseen manufacturing or distribution problems emerge.
The accelerated approval pathway for seniors means Moderna commits to conducting additional studies post-approval to confirm the vaccine's effectiveness in that population. Those studies would occur over subsequent years. If those studies confirm benefit, the accelerated approval converts to full approval. If they reveal problems, the FDA can revoke approval, though that's rare.
For younger seniors (ages 50-64), the pathway is straightforward. Full approval based on the Phase 3 trial data would presumably come alongside the accelerated approval decision. That population could access the vaccine immediately upon approval.
The broader question is whether the FDA maintains its timeline and doesn't get pulled back into political disputes. Prasad has control over whether the review progresses smoothly or encounters new obstacles. If he's genuinely willing to allow the application to move forward, the August timeline is achievable. If new objections emerge—or if new political pressure is applied—things could stall.
The Precedent: Why This Moment Matters Beyond Moderna
What happened with Moderna's mRNA flu vaccine establishes precedents that will shape how other vaccines are treated going forward.
First, it establishes that political appointees in the Trump administration can reject vaccine applications based on debatable scientific grounds, then reverse those decisions when companies propose alternative approaches. That changes how companies will craft applications and manage regulatory relationships.
Second, it signals that mRNA vaccines face higher scrutiny in the current regulatory environment. If Prasad rejected an mRNA vaccine for the flu—a disease where vaccines are well-established and needed—then all mRNA vaccine development faces elevated risk. Companies pursuing mRNA cancer vaccines, mRNA RSV vaccines, or other applications will factor in this regulatory uncertainty.
Third, it demonstrates that career scientists' advice can be overruled by political appointees. That's concerning because it introduces uncertainty into the review process. Career scientists can make their case, but their recommendations aren't dispositive. Political appointees can disagree and reject applications. That's demoralizing to the scientists and creates instability in the regulatory process.
Fourth, it shows that political pressure and negative publicity can prompt reversals. If Prasad hadn't reversed course, the criticism would have continued. By reversing, he defused immediate criticism, though questions remain about the decision's basis. That pattern—initial hardline stance, then reversal when faced with criticism—might repeat with other contested applications.
These precedents shape behavior throughout the industry. Vaccine companies will be more cautious about pursuing mRNA platforms. They'll anticipate regulatory obstacles. They'll plan for longer timelines and higher costs. That costs innovation and slows development.
From a public health perspective, the precedent is concerning. Vaccines develop faster and reach patients quicker when regulatory processes are predictable and science-driven. When political appointees can reject applications based on debatable grounds, then reverse when pressured, it creates instability. Companies can't plan effectively. Regulators can't maintain consistent standards. The process becomes less about science and more about political maneuvering.
This is the lasting damage from the Moderna situation, even with the reversal. The integrity of the regulatory process has been compromised. That affects how vaccines get developed, which vaccines get pursued, and how quickly life-saving vaccines reach patients.
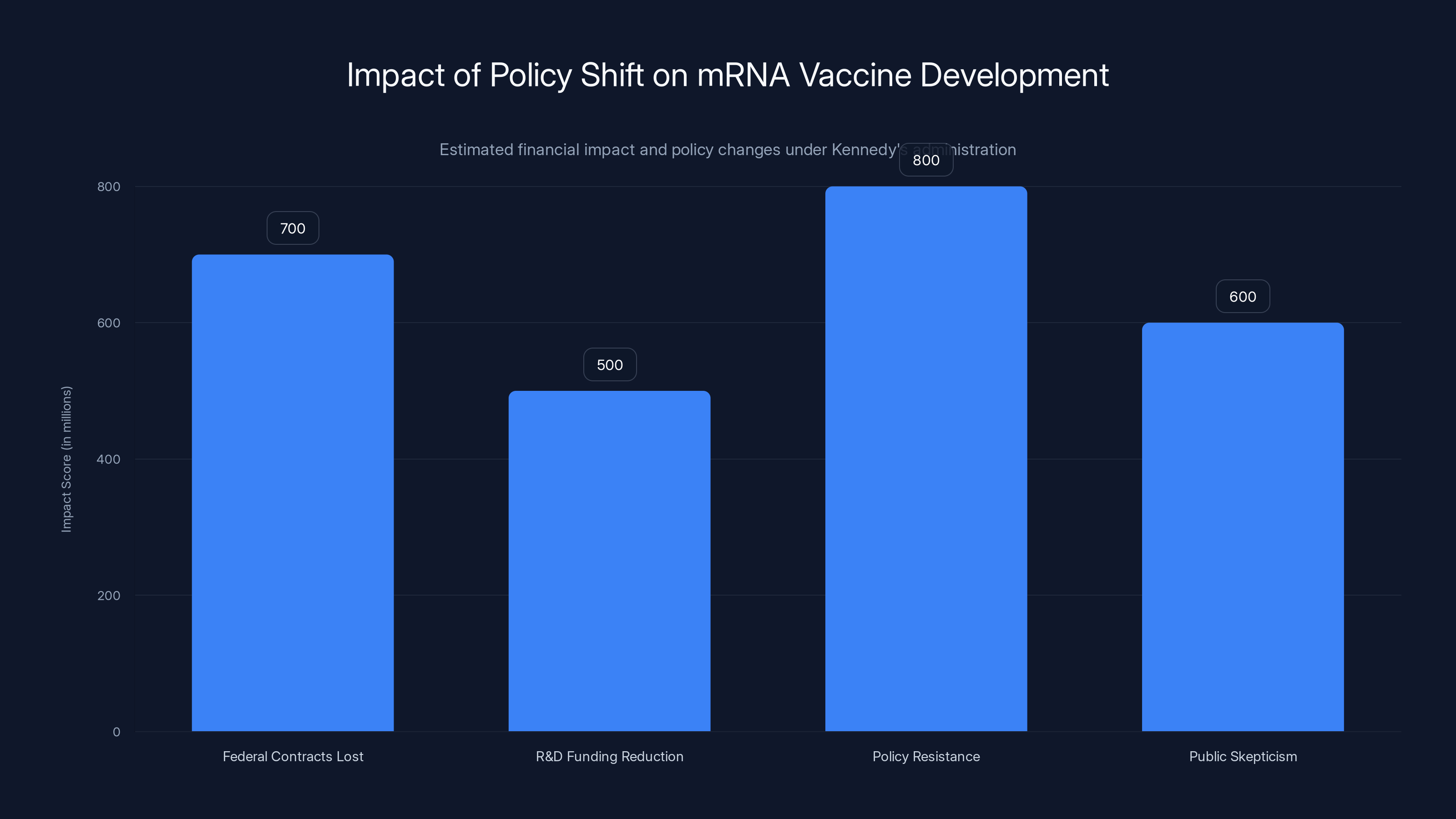
The shift in federal policy under Kennedy's administration has led to significant financial and ideological impacts on mRNA vaccine development, with an estimated $700 million loss in federal contracts.
Looking Forward: The Future of mRNA Vaccines and Vaccine Innovation
The Moderna situation occurs against the backdrop of broader questions about the future of mRNA vaccine technology and vaccine innovation generally.
Scientifically, mRNA vaccines represent a genuine breakthrough. They're faster to develop than traditional vaccines, easier to manufacture at scale, and effective against multiple diseases. The technology has proven itself with COVID-19 vaccines. The logical next step is to expand mRNA vaccines to other infectious diseases and potentially to cancers, leveraging the immune system to fight malignant cells.
But that scientific potential isn't automatically realized. Technology adoption depends on institutional support, funding, and regulatory clarity. The Trump administration is providing none of those things. Instead, it's actively hostile toward mRNA technology.
That hostility will have consequences. Researchers trained in mRNA vaccine development will move into other fields. Companies will reduce investment. Startups won't form because the regulatory environment is too uncertain. In five years, there will be fewer mRNA vaccines in development because the decisions being made today shaped that outcome.
Is that situation permanent? No. Future administrations can shift policy, restore funding, and signal support for mRNA vaccines. But there's inertia. When researchers leave a field, it takes time to rebuild expertise. When companies exit a space, they don't quickly re-enter. When funding dries up, research programs shutter. Recovery takes years, even with supportive policies.
The potential losses are significant. Imagine a future where mRNA cancer vaccines could be developed but weren't because funding evaporated in 2025. Imagine mRNA vaccines for malaria that never got pursued because the regulatory environment became hostile. Those losses are real even if they're not visible in the short term.
For Moderna specifically, the reversal is a reprieve. The mRNA flu vaccine will likely be approved and could reach the market in 2026. But the broader question of whether the company continues aggressive mRNA vaccine development—or scales back and explores alternative platforms—remains open. The answer probably depends on how subsequent regulatory interactions go.
What Happened to FDA's Scientific Independence?
One of the most concerning aspects of the Moderna situation is what it reveals about the FDA's scientific independence. The agency was created to make decisions based on evidence, not politics. Career scientists are protected from political pressure because their job is to evaluate data, not serve political objectives.
But when a political appointee can simply reject an application that career scientists recommend for review, that independence is compromised. It doesn't matter if the rejection is eventually reversed. The fact that it happened at all signals that scientific advice isn't determinative.
This creates a credibility problem. If the FDA is making decisions based on politics rather than science, how can the public trust those decisions? The whole point of FDA review is to provide independent scientific assessment. When that independence is compromised, the process loses legitimacy.
Moderna's situation isn't the only example. Throughout the Trump administration's health agencies, political appointees have overruled career scientists on various issues. Each instance chips away at the legitimacy of federal health agencies. Over time, the damage compounds. People stop trusting FDA approvals because they perceive politics in the decision-making. Companies lose confidence in regulatory predictability. The entire system becomes less effective.
Restoring scientific independence would require changes at the leadership level. It would require appointees who are willing to be bound by scientific evidence, even when that evidence contradicts their preferred policies. It would require protecting career scientists from retaliation when their recommendations contradict political appointees.
Whether that happens is an open question. What's clear is that the status quo—where political appointees can override scientists—is unsustainable for long-term institutional credibility.

The Broader Anti-Vaccine Agenda: Context for Vaccine Skepticism
Understanding the Moderna situation requires understanding the broader anti-vaccine agenda that the Trump administration is pursuing.
Robert F. Kennedy Jr. and his allies aren't primarily concerned with the trial design for Moderna's flu vaccine. That's a proxy battle. Their actual concern is that mRNA vaccines exist and are being developed. They want to slow, stop, or prevent mRNA vaccine development because they oppose the technology philosophically.
That opposition isn't grounded in evidence. Billions of mRNA vaccine doses have been administered globally. Safety profiles are well-established. Efficacy is documented. By every scientific standard, mRNA vaccines are safe and effective.
But Kennedy's movement isn't primarily interested in evidence. It's interested in narrative. The narrative is: "Vaccines are dangerous. mRNA vaccines are particularly dangerous. Regulators are captured by industry. You can't trust official health agencies." That narrative drives political support, funding, and institutional change.
The Moderna situation fits perfectly into that narrative. When the FDA rejects an mRNA vaccine application—even though career scientists recommended approval—it seems to confirm the narrative. "Look, regulators are so concerned about mRNA vaccines that they're rejecting them." The reversal is presented as: "They were forced to reverse because people noticed the injustice." The actual complexity—political appointees overruling scientists, then reversing when faced with criticism—gets lost.
What Kennedy's movement is pursuing is a comprehensive shift in vaccine policy. They want fewer vaccine recommendations, lower vaccination rates, and particular skepticism toward newer vaccine technologies. The Moderna situation is one battle in that broader campaign.
Vaccine companies understand this. They're not fighting over trial design details. They're fighting over whether their technology will be supported or opposed by federal regulators. That's an existential question for companies pursuing vaccine development.
The Industry Response: Vaccine Companies Retrench
Companies working on vaccine development are responding to the Moderna situation and the broader anti-vaccine environment by retreating from vaccine development.
Job cuts are happening across the industry. Researchers who specialized in vaccine development are being laid off or moved to other projects. Research programs that were developing new vaccines are being paused or cancelled. Investment in vaccine development is decreasing as companies reallocate capital to therapeutics or other areas that seem politically safer.
This is happening not because vaccines aren't medically important or scientifically promising. It's happening because the regulatory and political environment has become hostile. Companies can't develop vaccines in an environment where regulators might reject applications for political reasons. They can't invest in vaccine platforms when federal funding has evaporated. They can't justify expensive research programs when the return on investment is uncertain.
The consequence is a slowdown in vaccine innovation. The next generation of vaccines—for RSV, for malaria, for cancers, for emerging pathogens—won't be developed, or will be developed more slowly. That's bad for public health. It means conditions that could potentially be prevented or treated with new vaccines won't be addressed that way.
Smaller biotech companies pursuing vaccine development are facing particularly acute pressure. They often rely on federal funding and larger companies' partnerships to fund early-stage research. With federal funding declining and larger companies retrenching, smaller companies have nowhere to turn. Many will exit vaccine development entirely.
That consolidation around anti-vaccine policies has multiplier effects. As companies exit vaccine development, the field loses talent and expertise. Universities and research institutes reduce vaccine-focused programs because students and postdocs see fewer job opportunities. Funding agencies receive fewer grant applications for vaccine research because researchers know the political winds are unfavorable.
These structural changes are hard to reverse. Even if future administrations are supportive of vaccine development, rebuilding a field takes years. Researchers who left the field won't return immediately. Companies that exited the space won't re-enter quickly. The momentum is lost.

Public Health Implications: Who Bears the Costs?
When vaccine development slows, who bears the costs? Primarily, the public does.
Elderly people who could have been protected by new flu vaccines won't get those protections until later, if they're developed at all. People at risk from RSV might not have an effective vaccine option. People at risk from malaria might not get a vaccine that could have prevented the disease. These aren't theoretical problems. These are real diseases that affect millions of people.
There's also a pandemic preparedness angle. If the next novel pathogen emerges—a new coronavirus, a new influenza strain, something entirely unexpected—mRNA vaccine technology could be crucial for rapid vaccine development. But if mRNA vaccine development has been curtailed, if the companies pursuing mRNA vaccines have exited the field, if scientists trained in mRNA vaccine development have moved to other careers, we'll be starting from behind in a crisis.
Pandemic preparedness is inherently hard to justify politically because its benefits are hypothetical. You invest money and effort to prepare for something that you hope never happens. When that something doesn't happen, people ask why they invested in preparation. That makes pandemic preparedness politically vulnerable, especially to administrations skeptical of federal spending on public health.
The Moderna situation illustrates this dynamic. The company was maintaining pandemic preparedness capacity—ability to rapidly develop and produce vaccines if needed—that the Trump administration defunded. That saves money in the short term. It creates risk in the long term. When the next pandemic happens, that risk becomes visible. But by then, it's too late to rebuild the capacity that was eliminated.
There's also a question about trust. When people lose confidence in federal health agencies—because they perceive politics in vaccine approvals—that affects public health broadly. If vaccine recommendations become seen as politically motivated rather than scientifically grounded, vaccination rates drop. That puts vulnerable populations at risk.
The Moderna situation, even with the reversal, damages trust in FDA vaccine reviews. The public sees that a political appointee rejected an application that scientists supported. That confirms suspicions that politics, not science, drives regulatory decisions. Even though the FDA reversed course, the damage to confidence has occurred.
The Path Forward: What Needs to Happen
If the goal is to protect vaccine innovation, restore FDA scientific independence, and maintain public trust in vaccine approval processes, several things need to change.
First, there needs to be protection for career scientists. They should be insulated from political pressure. Their recommendations should carry weight in decision-making. When scientists recommend that an application move forward, political appointees should have a high bar for overruling them. They should need clear scientific justification, not debatable objections used as cover for political goals.
Second, there needs to be restoration of federal funding for vaccine development and pandemic preparedness. Companies can't maintain vaccine development programs without economic support. Federal funding serves that role. Maintaining pandemic preparedness capacity is a legitimate public health investment, even when it doesn't produce immediate returns.
Third, there needs to be transparent decision-making. When regulatory decisions are made, the reasoning should be clearly explained. If there are legitimate scientific concerns about an application, those concerns should be articulated explicitly. If decisions are reversed, the explanation should address what changed and why.
Fourth, there needs to be accountability for leadership appointments. The people installed in federal health agencies should have relevant expertise and commitment to scientific evidence. When appointments are made to pursue political agendas, it compromises agencies' missions.
Fifth, there needs to be communication to industry about what regulatory standards actually are. Companies need to know what they need to accomplish to get approval. When standards shift based on political winds, companies can't plan effectively. Clarity and consistency matter.
These changes would require political will. They would require leaders who prioritize scientific integrity over political goals. Whether that's forthcoming is unclear. What's clear is that the status quo—where political appointees override scientists and regulatory decisions lack transparency—isn't sustainable.

Conclusion: A Turning Point for Vaccine Development and Public Trust
The FDA's rejection of Moderna's mRNA flu vaccine, and the subsequent reversal, represents a turning point in how vaccines are regulated in the United States. It exposed tensions between political appointees and career scientists. It revealed the vulnerability of vaccine approval processes to political pressure. It signaled to the entire vaccine industry that the regulatory environment has shifted in ways that make certain technologies less viable.
On the surface, the reversal is positive. Moderna's vaccine will get reviewed. It will likely be approved. Seniors will have access to a new flu vaccine option by late 2026. The immediate outcome seems acceptable.
But the larger story is concerning. A political appointee rejected an application that scientists supported, using questionable reasoning. The decision was reversed not because the science changed but because Moderna proposed a different regulatory pathway and political pressure mounted. That sequence erodes confidence in the regulatory process.
The reversal also came with costs. Moderna had to accept accelerated approval for seniors instead of full approval. That's not catastrophic, but it's not ideal either. The company had to adapt its strategy in response to political obstacles, rather than having its application processed on scientific merit.
More broadly, the situation sends signals throughout the vaccine industry that mRNA technology is politically disfavored. Companies are responding by reducing investment in mRNA vaccine development. Researchers are moving to other fields. Funding is drying up. The momentum that existed around mRNA vaccines, particularly for applications beyond COVID-19, is dissipating.
The long-term consequences won't be visible for years. A researcher deciding today not to pursue mRNA vaccine development means no breakthrough in that area in five years. A company deciding to pause an mRNA vaccine program means that program never reaches patients. A venture capital firm reducing vaccine investments means fewer startups pursuing vaccine innovation.
Whether this trajectory continues depends on whether future regulatory interactions follow the same pattern—political obstacles, then reversals when pressured—or whether the precedent established by the Moderna situation becomes the normal operation of vaccine review.
What's certain is that the episode revealed deep fissures in the vaccine approval process. Political appointees and career scientists are at odds. Trust in FDA independence is damaged. The industry is retrenching. Public confidence in vaccine regulation is declining.
These are serious problems that extend far beyond Moderna's flu vaccine. They affect how future vaccines get approved, which vaccines get developed, and whether the United States maintains leadership in vaccine innovation. The Moderna situation was one battle in a larger conflict over vaccine policy. How that conflict resolves will shape vaccine development for years to come.
FAQ
What is an mRNA vaccine and how does it work?
mRNA vaccines introduce synthetic messenger RNA into cells, instructing them to produce a specific protein that triggers immune responses. The immune system recognizes this protein as foreign and develops antibodies and immune cells to fight it. This approach is faster to develop and manufacture than traditional vaccines, which is why it was valuable during the COVID-19 pandemic and remains promising for other diseases.
Why was the FDA's initial rejection of Moderna's vaccine application controversial?
The rejection was controversial because it appeared to be motivated by politics rather than science. Vinay Prasad, a political appointee, overruled career FDA scientists who had recommended reviewing the vaccine application. His stated objection about the trial's control vaccine was debatable—the FDA had previously approved the trial design—and when Moderna proposed a different regulatory pathway, the FDA reversed course. This suggested the objection may have been a pretext.
What is accelerated approval and why did Moderna accept it?
Accelerated approval is a regulatory pathway that allows products to reach patients faster while the manufacturer commits to conducting additional studies after approval to confirm effectiveness. Moderna accepted it for seniors aged 65+ because it was better than the alternative of having its application rejected. The accelerated pathway means the vaccine can reach older adults sooner, even though additional studies will be required post-approval.
How did the Trump administration's vaccine policies contribute to this situation?
Robert F. Kennedy Jr., the Secretary of Health and Human Services, has an explicit anti-vaccine agenda and has installed allies in key positions throughout federal health agencies. Vinay Prasad, who rejected Moderna's application, appears to be aligned with this anti-mRNA vaccine agenda. The administration has also defunded pandemic vaccine development programs and cut $700 million in federal contracts from Moderna, creating an environment hostile to mRNA vaccine development.
What does this mean for vaccine innovation going forward?
The Moderna situation signals to the vaccine industry that mRNA vaccines face regulatory and political obstacles. Companies are responding by reducing investment in vaccine development, laying off researchers, and pausing research programs. Over time, this means fewer new vaccines will be developed, research progress will slow, and pandemic preparedness capacity will diminish. The consequences won't be immediately visible but will become apparent over years as promising vaccine projects never reach patients.
Is there scientific evidence that mRNA vaccines are unsafe?
No. Billions of mRNA vaccine doses have been administered globally, particularly for COVID-19. Extensive post-authorization safety monitoring shows that serious adverse events are extraordinarily rare. The most common side effects are mild and temporary, such as arm soreness and fatigue. Claims that mRNA vaccines cause rising rates of chronic disease are not supported by epidemiological evidence. The skepticism toward mRNA vaccines in the Trump administration appears ideological rather than evidence-based.
When will Moderna's flu vaccine be available if approved?
If approved by the August 5, 2026 decision date, Moderna expects the mRNA flu vaccine to be available to seniors by late 2026. Full approval is expected for people aged 50-64 based on Phase 3 trial data. Accelerated approval for people 65+ would require additional post-approval studies to confirm effectiveness in that age group before conversion to full approval.
How does this FDA reversal affect trust in vaccine approvals?
The reversal damages trust because it shows that vaccine approval decisions can be influenced by political pressure rather than determined solely by scientific merit. Career scientists recommended reviewing Moderna's application, but a political appointee rejected it. When the rejection became publicly controversial, the FDA reversed course. This pattern suggests that regulatory decisions might be driven by politics, which undermines public confidence in the FDA's scientific independence. Even though the outcome appears acceptable, the process that led to it raises concerns about how future vaccines will be evaluated.

Key Takeaways
- Political appointee Vinay Prasad rejected Moderna's mRNA flu vaccine application despite FDA career scientists recommending review, citing disputed concerns about the trial's control vaccine
- FDA reversed the rejection within days after Moderna proposed splitting the application into separate regulatory pathways for different age groups
- The reversal signals that regulatory decisions may be driven by political pressure rather than scientific merit, damaging trust in FDA independence
- Trump administration cuts totaling $700 million in federal vaccine contracts have caused Moderna and other vaccine companies to reduce mRNA vaccine development programs
- Vaccine industry is retreating from mRNA vaccine development due to regulatory uncertainty, federal funding cuts, and anti-vaccine sentiment in the Trump administration's health agencies
- Career FDA scientists argued that the trial design was acceptable and that Moderna's vaccine application should move forward, but were overruled by a political appointee
- Moderna will likely gain approval by August 5, 2026, with the vaccine potentially available to seniors by late 2026 under the accelerated approval pathway
- The situation reveals deep tensions between political appointees and career scientists at the FDA, raising questions about the integrity of the vaccine approval process
Related Articles
- FDA Vaccine Official Overruled Scientists on Moderna Flu Shot [2025]
- Ivermectin as Cancer Treatment: Why Federal Research Funding Raises Red Flags [2025]
- HHS AI Tool for Vaccine Injury Claims: What Experts Warn [2025]
- Why Microdosing LSD Fails for Depression: The Placebo Study [2025]
- Microdosing for Depression: Placebo Effect vs. Real Benefits [2025]
- US Exit From WHO: $768 Million Gap & Global Health Crisis [2025]
![FDA Reverses Moderna mRNA Flu Vaccine Rejection: Inside the Political Turmoil [2025]](https://tryrunable.com/blog/fda-reverses-moderna-mrna-flu-vaccine-rejection-inside-the-p/image-1-1771436395565.jpg)



